Neuroform Atlas® Stent System – P180031/S001
This is a brief overview of information related to FDA’s approval to expand the indications for use to market this product. See the links below to the Summary of Safety and Effectiveness Data (SSED) and product labeling for more complete information on this product, its indications for use, and the basis for FDA’s approval.
Product Name: Neuroform Atlas® Stent System
PMA Applicant: Stryker Neurovascular
Address: 47900 Bayside Parkway, Fremont, CA 94538
Approval Date: July 30, 2020
Approval Letter: Approval Order
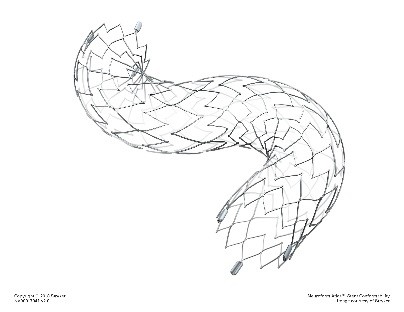
What is it? The Neuroform Atlas Stent System is intended to hold in place coil devices used to plug up aneurysms in the brain that have a neck size greater than or equal to 4 mm or a dome-to-neck ratio less than 2 (wide-necked brain aneurysms). The Neuroform Atlas Stent System consists of a self-expandable metal (nitinol) tube-shape device (stent) and a wire to place it inside a brain artery.
How does it work? A physician inserts the delivery wire containing the Neuroform Atlas stent with a small catheter through a cut into a large artery in the groin (femoral artery). The Neuroform Atlas stent is carefully guided to the aneurysm and permanently implanted to hold in place embolization coils implanted in the sac of the aneurysm.
When is it used? The Neuroform Atlas Stent System is indicated for use with neurovascular embolization coils in the anterior and posterior circulation of the brain for patients 18 years of age or older with saccular wide-necked brain aneurysms arising from an artery (parent vessel) with a diameter of 2.0 mm or greater and less than or equal to 4.5 mm.
What will it accomplish? The Neuroform Atlas Stent System is intended to assist in blocking off the aneurysm, preventing further growth or rupture.
The Neuroform Atlas Stent System was reviewed and approved under P180031 for use in wide-necked aneurysms in the anterior circulation of the brain. This supplement, P180031/S001, provided additional clinical data to expand the indications for use to include wide-necked brain aneurysms located in the posterior circulation of the neurovasculature.
In a clinical study of 124 patients with posterior circulation wide-necked brain aneurysms treated with the Neuroform Atlas Stent System, 77% of the treated brain aneurysms were completely sealed off at one year without the need for re-treatment or significant narrowing of the parent artery (stenosis). Of the patients treated in the study, 18.1% experienced serious side effects, including death, stroke, or brief blockage of the blood supply (transient ischemic attack or TIA).
When should it not be used? The Neuroform Atlas Stent System should not be used in patients who:
- Have a parent vessel size that does not fall within the indicated range;
- Are contraindicated for anticoagulant and anti-platelet therapy;
- Have not received anti-platelet agents prior to stent implantation;
- Have brain blood vessels that are not appropriate for endovascular treatment due to conditions such as severe intracranial vessel tortuosity or stenosis or intracranial vasospasm not responsive to medical therapy;
- Have an active bacterial infection;
- Have a pre-existing stent in place in the parent artery at the target intracranial aneurysm location.
Because of the risk of stroke and death associated with placement of the device, patients and physicians should carefully weigh the benefits versus the risks of device treatment for each individual patient based on their medical health status and risk factors for brain aneurysm rupture during their expected lifetime such as age, comorbidities, history of smoking, brain aneurysm size, location and morphology, family history, history of prior asymptomatic subarachnoid hemorrhage, documented growth of brain aneurysm on serial imaging, presence of multiple brain aneurysms, and presence of concurrent diseases. The benefits may not outweigh the risks associated with device use in certain patients; therefore, careful patient selection is recommended based on clinical practice guidelines or tools to assess the lifetime risk of brain aneurysm rupture.
Additional information (including warnings, precautions, and adverse events):
Summary of Safety and Effectiveness Data (SSED) and labeling are available at: