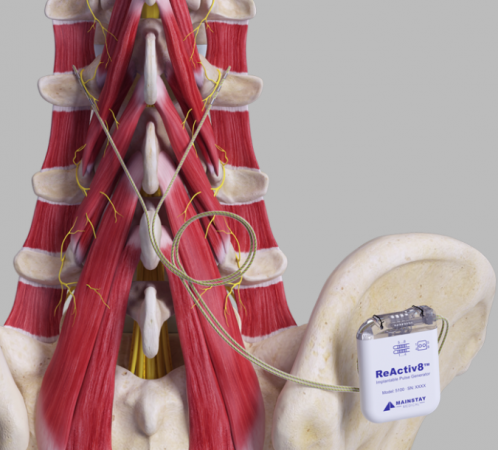
Reactiv8 Implantable Neurostimulation System - P190021
This is a brief overview of information related to FDA’s approval to market this product. See the links below to the Summary of Safety and Effectiveness Data (SSED) and product labeling for more complete information on this product, its indications for use, and the basis for FDA’s approval.
Product Name: ReActiv8 Implantable Neurostimulation System
P190021 Applicant: Mainstay Medical Limited
Address: 6601 Shingle Creek Parkway, Suite 200, Brooklyn Center, MN, 55430
Approval Date: June 16, 2020
Approval Letter: Approval Order
What is it? The ReActiv8 Implantable Neurostimulation System includes an implantable pulse generator (IPG), two stimulation leads, a magnet, and a wireless remote. The IPG delivers electrical stimulation pulses to certain nerves responsible for activating the lumbar multifudus muscle, the key muscles responsible for stabilizing the lower back.
How does it work? A doctor implants the ReActiv8 IPG in the patient’s lower back and a stimulation lead is placed on the lumbar multifudus muscle on the left and right side of the patient’s spine. The IPG delivers electrical stimulation pulses to the nerves that activate the lumbar multifudus muscle, which can lead to improvement of chronic lower back pain. Using the wireless remote, the patient is able to start, pause, or stop a session of electrical stimulation.
When is it used? The ReActiv8 Implantable Neurostimulation System is intended to help with the management of chronic low back pain associated with the muscular weakness of the lumbar multifidus muscle in patients who have failed therapy including pain medications and physical therapy and are not candidates for spine surgery. Before implanting the device, multifudus muscle atrophy and weakness must be shown using magnetic resonance imaging (MRI) or during a physical exam using the prone instability test.
What will it accomplish? The ReActiv8 Implantable Neurostimulation System can decrease chronic lower back pain in patients that have weakness of the lumbar multifudus muscle. In a clinical study, the majority of patients that had the ReActiv8 Implantable Neurostimulation System demonstrated significant improvement in pain symptoms after 120 days as compared to patients who had a device in which sub-optimal stimulation was provided. When considering all patients who received the device, the patients with active stimulation demonstrated greater reduction in pain scores. In addition, patients with the device also experienced clinically meaningful improvements in function after 120 days as demonstrated by the Oswestry Disability Index.
When should it not be used? The ReActiv8 Implantable Neurostimulation System should not be used in patients who are:
- Unable to operate the system
- Unable to have the surgery
Additional information (including warnings, precautions, and adverse events):