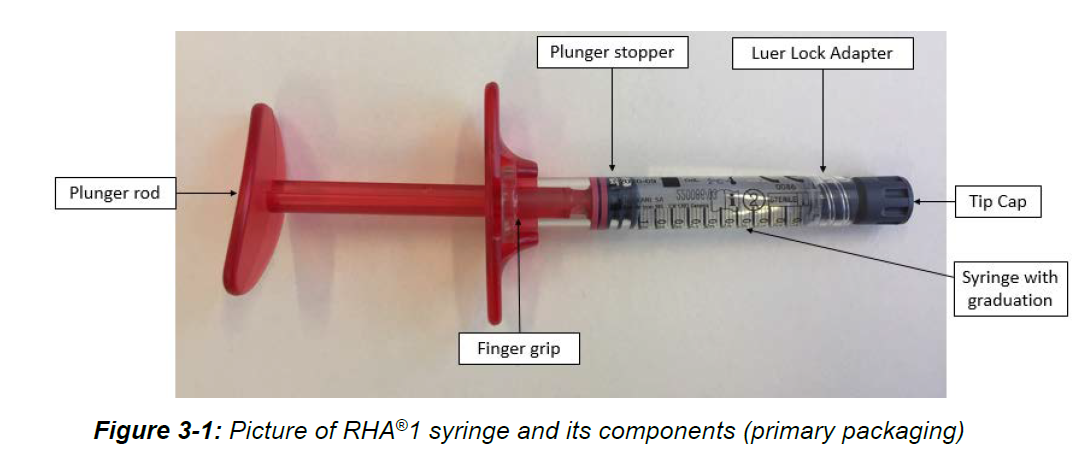
RHA Redensity – P170002/S012
This is a brief overview of information related to FDA’s approval to market this product. See the links below to the Summary of Safety and Effectiveness Data (SSED) and product labeling for more complete information on this product, its indications for use, and the basis for FDA’s approval.
Product Name: RHA Redensity
PMA Applicant: TEOXANE S.A.
Address: Les Charmilles, Rue de Lyon, 105, CH - 1203 Geneva, Switzerland
Approval Date: 12/22/2021
Approval Letter: Approval Order
What is it?
RHA Redensity is a gel implant or dermal filler that is injected in specific areas of facial tissue to reduce the appearance of lines and wrinkles. It consists of the chemical sodium Hyaluronic Acid (NaHA), 1,4-butanediol diglycidyl ether (BDDE) and 0.3% of the drug lidocaine hydrochloride to reduce pain on injection.
This approval is for the correction of moderate to severe vertical wrinkles around the mouth (dynamic perioral rhytids).
How does it work?
A doctor injects RHA Redensity into the dermis and superficial dermis for the correction of moderate to severe dynamic perioral rhytids.
When is it used?
RHA Redensity may be used to reduce moderate to severe wrinkles around the mouth of adults 22 years of age and older.
What will it accomplish?
RHA Redensity may help to reduce moderate to severe dynamic perioral rhytids for up to 12 months. In a clinical study, patients needed one or two treatments with multiple injections to achieve the best outcome. Specifically, 121 of the 150 subjects (80.7%) had a clinically meaningful improvement in their perioral wrinkles at the 8-week primary endpoint. Effectiveness was seen in 66.5% of subjects at 12 months.
Common side effects include:
- Bruising
- Discoloration
- Firmness
- Itching
- Lumps/Bumps
- Pain
- Redness
- Swelling
- Tenderness
When should it not be used?
RHA Redensity should not be used in patients who have:
- A history of a severe hypersensitivity (anaphylaxis)
- A history or presence of multiple severe allergies
- A known hypersensitivity to gram positive bacterial proteins
- A history of allergies to lidocaine or other amide anesthetics
- Bleeding disorders
Additional information (including warnings, precautions, and adverse events):
Other: