SoftVue Automated Whole Breast Ultrasound System with Sequr Breast Interface Assembly – P200040
This is a brief overview of information related to FDA’s approval to market this product. See the links below to the Summary of Safety and Effectiveness Data (SSED) and product labeling for more complete information on this product, its indications for use, and the basis for FDA’s approval.
Product Name: SoftVue Automated Whole Breast Ultrasound System with Sequr Breast Interface Assembly
PMA Applicant: Delphinus Medical Technologies, Inc.
Address: 45525 Grand River Avenue, Novi, MI 48374 USA
Approval Date: October 6, 2021
Approval Letter: Approval Order
What is it?
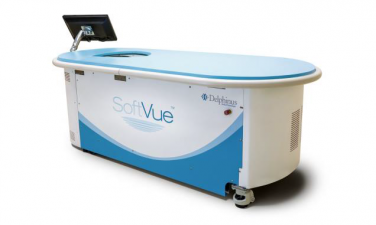
The SoftVue Automated Whole Breast Ultrasound System with Sequr Breast Interface Assembly (SoftVue System) is a medical device for three-dimensional, automated ultrasound scanning of the whole breast of patients with dense tissue.
How does it work?
The SoftVue System includes a table for the patient to lie face down, with their breast positioned in a water tank that is a part of the table. The ultrasound probes form a circle around the breast, moving up and down, to allow for scanning of the whole breast.
When is it used?
The SoftVue system is used with mammography for breast cancer screening in asymptomatic patients who have mostly or almost dense tissue according to the Breast Imaging and Reporting Data System or BI-RADS.
What will it accomplish?
The SoftVue System is intended to help increase breast cancer detection in patients who are asymptomatic and have dense breasts.
When should it not be used?
There are no known contraindications.
Additional information (including warnings, precautions, and adverse events):
The Summary of Safety and Effectiveness Data (SSED) and labeling are available at:
Other: