SYNERGY Everolimus-Eluting Platinum Chromium Coronary Stent System (Monorail and Over-The-Wire) – P15003/S058
This is a brief overview of information related to FDA’s approval to market this product. See the links below to the Summary of Safety and Effectiveness Data (SSED) and product labeling for more complete information on this product, its indications for use, and the basis for FDA’s approval.
Product Name: SYNERGY™ Everolimus-Eluting Platinum Chromium Coronary Stent System (Monorail™), SYNERGY™ Everolimus-Eluting Platinum Chromium Coronary Stent System (Over-The-Wire™), and SYNERGY™ XD Everolimus-Eluting Platinum Chromium Coronary Stent System (Monorail™)
PMA Applicant: Boston Scientific Corporation
Address: One Scimed Place, Maple Grove, MN 55311-1566
Approval Date: August 11, 2020
Approval Letter: Approval Order
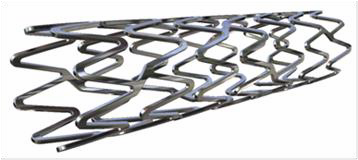
What is it? The SYNERGY Everolimus-Eluting Platinum Chromium Coronary Stent Systems are intended to treat a narrowed blood vessel (coronary artery) caused by coronary artery disease. The system consists of a catheter delivery system and a platinum-chromium metal stent. The stent is coated with the drug everolimus and a thin coating of poly(lactic-co-glycolic acid) (PLGA), a material that dissolves into the body.
How does it work? A physician inserts the stent’s delivery balloon catheter into a blood vessel in the patient’s arm or groin. The stent is then positioned at the site of the coronary artery and the balloon is inflated, which expands the stent and presses it against the coronary artery wall. The stent remains permanently implanted within the coronary artery to help keep it open and improving the supply of blood and oxygen to the heart. The drug (everolimus) is released over time from the SYNERGY Stent surface into the coronary artery wall to help prevent re-narrowing, which sometimes occurs after the stent is implanted.
When is it used? The SYNERGY Stent is indicated for use in patients who have a narrowing in their coronary arteries and are at high risk for bleeding due to previous conditions including, but not limited to, advanced age, the need for chronic or lifelong anticoagulation (aspirin and other blood thinners), history of major bleeding, history of stroke, and renal insufficiency.
This approval expands the indications for use to include patients at high risk for bleeding.
What will it accomplish? In a clinical study, a total of 1,487 patients at high risk for bleeding were treated using the SYNERGY stent and were able to stop taking one of their two blood thinner medications (antiplatelet therapy) after three months. The results demonstrated that patients with the stent who stopped taking one blood thinner after three months but kept taking aspirin were not at increased risk of death or heart attack (myocardial infarction) in the first year. Additionally, these patients had a similar frequency of blood clots forming blockages inside the stent (rate of stent thrombosis) during the first year compared to reports for other drug eluting stents.
When should it not be used? The SYNERGY Everolimus-Eluting Platinum Chromium Coronary Stent System should not be used with:
- Patients with uncorrected bleeding disorders or patients who cannot receive anticoagulation or antiplatelet aggregation therapy
- Patients with known hypersensitivity or allergies to:
- 316L stainless steel, or platinum, chromium, iron, nickel or molybdenum
- Everolimus or structurally-related compounds
- The PLGA polymer or its individual components
- Patients who cannot have complete inflation of an angioplasty balloon or proper placement of the stent or delivery device
Additional information (including warnings, precautions, and adverse events):