TransPyloric Shuttle/TransPyloric Shuttle Delivery Device - P180024
This is a brief overview of information related to FDA's approval to market this product. See the links below to the Summary of Safety and Effectiveness Data (SSED) and product labeling for more complete information on this product, its indications for use, and the basis for FDA's approval.
Product Name: TransPyloric Shuttle/TransPyloric Shuttle Delivery Device
PMA Applicant: BAROnova, Inc.
Address: 1551 Industrial Road, San Carlos, CA 94070
Approval Date: April 16, 2019
Approval Letter: Approval Order
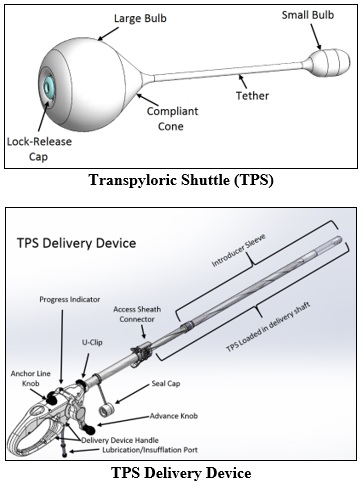
What is it? The TransPyloric Shuttle (TPS) is a weight-loss system that uses a removable gastric balloon that affects flow out of the stomach. The TPS is temporary and should be removed after 12 months.
How does it work? The TPS is placed into the patient's stomach through their mouth during a minimally invasive endoscopic procedure. Once in place, the TPS is formed using the TPS Delivery Device. The TPS forms a smooth large bulb connected to a smaller bulb by a flexible silicone tether.The large bulb remains in the stomach and the small bulb is designed to remain either in the stomach or cross the stomach into the small intestine to slow the time it takes for food to leave the stomach and enter the small intestine (gastric emptying). The TPS remains in the stomach for up to 12 months to help patients lose weight.
When is it used? The TransPyloric Shuttle/TransPyloric Shuttle Delivery Device is used in obese adult patients with a Body Mass Index (BMI) of 35.0-40.0 kg/m2 or a BMI of 30.0 to 34.9 kg/m2 with an associated medical condition (for example, diabetes) who have been unable to lose weight diet and behavior modification program, and exercise. It is intended to be used while a patient participates in a diet and exercise plan supervised by a health care provider.
What will it accomplish? During the clinical study, the TransPyloric Shuttle/TransPyloric Shuttle Delivery Device was implanted in patients for up to one year. Sixty-six percent of treated patients observed at least a 5% reduction in their total body weight compared to 30% of patients who did not receive the device but underwent the procedure for placement of the device. Treated patients lost an average of 9.3% of their body weight; and patientswho underwent the procedure but did not receive the device lost 2.8% of their body weight.
When should it not be used? The TPS device should not be used in patients if any of the following apply:
- Prior surgery or endoscopic intervention that has altered esophageal, gastric or small intestine (duodenal) anatomy
- Structural abnormality in the esophagus or pharynx
- Esophageal abnormality
- Patulous gastroesophageal junction
- Structural or functional disorders of the stomach including, stomach cannot empty itself of food in a normal fashion,, gastric ulcer, gastric mass, inflamed lining of the stomach, gastric varices, hiatal hernia (> 4cm), narrowing of the opening from the stomach, or any other disorder of the stomach
- Inflammatory and other conditions of the gastrointestinal (GI) tract, such as Crohn's disease
- Untreated Helicobacter pylori infection
- Active stomach or intestine ulcers
- Continuous use of aspirin, or nonsteroidal anti-inflammatory drugs
- Bleeding disorder or on blood thinners or medicine that stops blood clots
- Unable or unwilling to take proton pump inhibitors (PPI) medication for the duration of the device implant
- History of increase in the blood pressure within the veins of the liver, cirrhosis, and/or esophageal varices
- Diagnosis of bulimia or binge eating disorder or other severe psychiatric disorders
- Pregnancy or planned pregnancy in next 12 months
- Known or suspected allergic reaction to any materials in the device
- Any other medical condition that would not permit endoscopy or anesthesia
Additional information (including warnings, precautions, and adverse events):
- Summary of Safety and Effectiveness Data (SSED)
- Patient Labeling: Patient Information Booklet for the TransPyloric Shuttle® (TPS®)
- Physician Labeling: Transpyloric Shuttle/Transpyloric Shuttle Delivery Device Instructions For Use
- PMA Database Entry
Other Resources: