TREO® Abdominal Stent-Graft System - P190015
This is a brief overview of information related to FDA’s approval to market this product. See the links below to the Summary of Safety and Effectiveness Data (SSED) and product labeling for more complete information on this product, its indications for use, and the basis for FDA’s approval.
Product Name: TREO® Abdominal Stent-Graft System
PMA Applicant: Bolton Medical, Inc.
Address: 799 International Parkway, Sunrise, FL 33325
Approval Date: 05/04/2020
Approval Letter: Approval Order
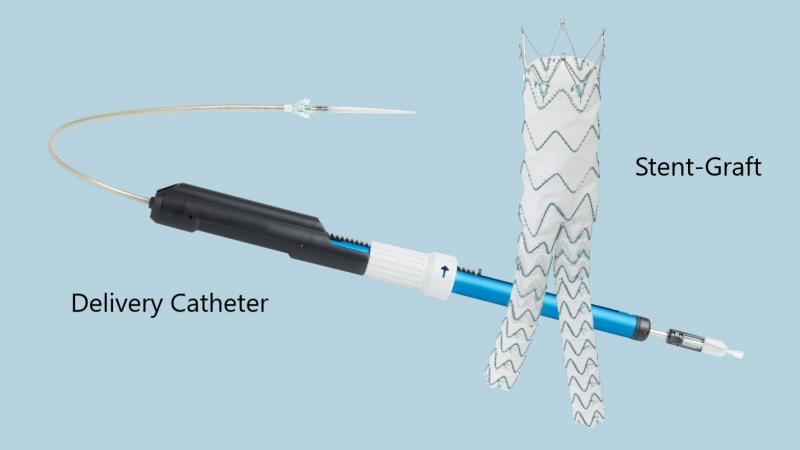
What is it? The TREO® Abdominal Stent-Graft System is used to repair a weakened and bulging section (aneurysm) of the aorta (largest artery in the abdomen) below the renal arteries.
The system consists of a tube-shaped implant (nitinol, polyester, and platinum-iridium) and a delivery catheter.
How does it work? The delivery catheter containing the stent graft is inserted into the femoral artery (a blood vessel in the upper leg) through a small cut. The delivery system is carefully guided to the site of the aneurysm in the abdominal aorta and placed to provide a new flow path for blood flow. This relieves the pressure on the weakened and bulging section of the aorta (aneurysm), which may prevent further aneurysm growth, bursting (rupture) of the aneurysm, and death.
When is it used? The TREO® Abdominal Stent-Graft System is used in patients who have an abdominal aortic aneurysm below the renal arteries.
What will it accomplish? The TREO® Abdominal Stent-Graft System is intended to prevent further growth or rupture of the aneurysm.
In a clinical study of 150 patients, 93% of patients were successfully treated without device- or aneurysm-related complications up to one year after the procedure.
When should it not be used? The TREO® Abdominal Stent-Graft System should not be used in patients with:
- Known allergies or sensitivities to the device materials (nitinol, polyester, and platinum-iridium).
- A condition that threatens to infect the graft.
Additional information (including warnings, precautions, and adverse events): Summary of Safety and Effectiveness Data (SSED) and labeling are available at:
Other: