Tula® System - P190016
This is a brief overview of information related to FDA’s approval to market this product. See the links below to the Summary of Safety and Effectiveness Data (SSED) and product labeling for more complete information on this product, its indications for use, and the basis for FDA’s approval.
Product Name: Tula® System
PMA Applicant: Tusker Medical
Address: 155 Jefferson Drive, Suite 200, Menlo Park, CA, 94025
Approval Date: November 25, 2019
Approval Letter: Approval Order
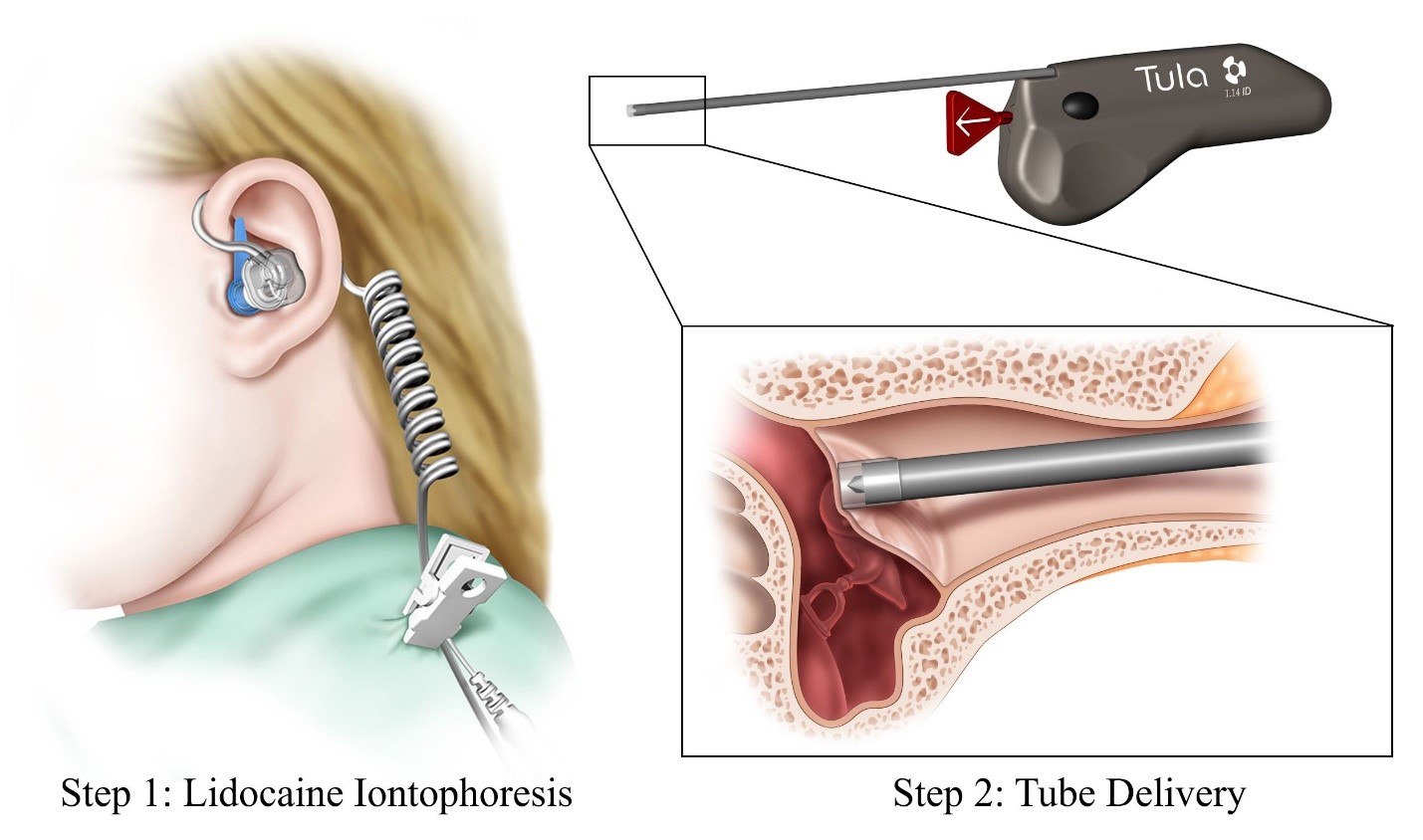
What is it? The Tula® System is intended to insert ear tubes (tympanostomy tubes) into the eardrum to treat repeated ear infections (recurrent acute otitis media) or fluid in the ear (otitis media with effusion) in young children and adults using local anesthesia in a physician’s office. The Tula® System consists of the Tula Iontophoresis System and the Tula Tube Delivery System. The Tula Iontophoresis System, which includes individually-fitted disposable ear plugs and ear sets, delivers a local anesthetic solution, TYMBION™, to the eardrum resulting in numbness of the eardrum. The Tula Tube Delivery System is then used to place the ear tube in the eardrum.
How does it work? The Tula Iontophoresis System uses a small electrical current to deliver TYMBION™ to the eardrum prior to tube insertion. The fitted earplugs keep the local anesthetic solution in the ear canal. Once the eardrum is adequately numb, the physician removes the earplugs and uses the Tula Tube Delivery System to create a small hole in the eardrum and insert the ear tube.
When is it used? Healthcare providers may recommend ear tubes as a treatment for repeated middle ear infections or persistent fluid in the middle ear. The Tula System offers physicians, patients, parents, and caregivers an alternative to the conventional hospital or surgical center-based procedure, which requires the administration of general anesthesia or sedation for insertion of ear tubes in pediatric (age 6 months and older) and adult patients.
What will it accomplish? The effectiveness of the Tula System for the delivery of ear tubes was assessed in a clinical study of 222 pediatric patients, ages 6 months to 12 years. Physicians were successful in placing ear tubes using the Tula System in 86% (103/120) of children ages 6 months to 4 years and in 89% (91/102) of children ages 5 to 12 years.
In the same study, pain and distress related to the procedure were evaluated. A video-based pain scoring method was used for younger children, ages 6 months to 4 years. Older children, ages 5 to 12 years, self-reported pain scores. For all patients, pain and distress were rated using a 0 to10 scale, where 10 represents severe pain or distress, and 0 represents no pain or distress. Children ages 6 months to 4 years had an average observed score of 4.0 for the ear tube insertion phase of the procedure, which decreased to 1.3 at the end of the procedure. Children ages 5 to12 years with successful ear tube insertion reported an average score of 3.3 for the tube insertion phase of the procedure, which decreased to 1.7 at the end of the procedure.
When should it not be used? The Tula® System should not be used in patients with any of the following conditions:
- An ear drum that is unusually thin, has a hole in it, or has not completely healed from a previous condition
- Injuries or cuts in the ear canal
- Abnormal blood vessels behind the ear drum
- An infection in the ear canal, referred to as otitis externa or swimmer’s ear
- Implantable electronic medical support systems, such as pacemakers, defibrillators, or cochlear implants
- A history of developing an allergic reaction to local anesthetic, or numbing, medications, or to any of the added TYMBION™ ingredients
- A family history of insensitivity to the numbing action of local anesthetic medications, including lidocaine
- Unusual ear anatomy that would prevent ear tube placement in the desired location
Additional information (including warnings, precautions, and adverse events):