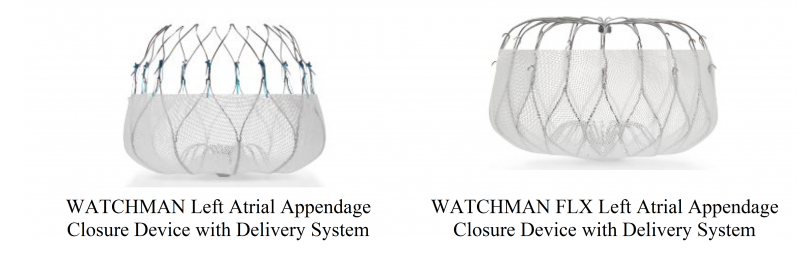
WATCHMAN Left Atrial Appendage Closure Device with Delivery System and WATCHMAN FLX Left Atrial Appendage Closure Device with Delivery System - P130013/S035
This is a brief overview of information related to FDA’s approval to market this product. See the links below to the Summary of Safety and Effectiveness Data (SSED) and product labeling for more complete information on this product, its indications for use, and the basis for FDA’s approval.
Product Name: WATCHMAN Left Atrial Appendage Closure Device with Delivery System and WATCHMAN FLX Left Atrial Appendage Closure Device with Delivery System
PMA Applicant: Boston Scientific Corporation
Address: Three Scimed Place, Maple Grove, MN 55331-1566
Approval Date: July 21, 2020
Approval Letter: Approval Order
What is it? The WATCHMAN Left Atrial Appendage Closure Device with Delivery System (WATCHMAN device) and WATCHMAN FLX Left Atrial Appendage Closure Device with Delivery System (WATCHMAN FLX device) are permanently implanted devices intended to prevent blood clots in the left atrial appendage (LAA) from entering the bloodstream. Both devices are made of a Nitinol (nickel-titanium) frame with an attached fabric cap. The WATCHMAN device and the WATCHMAN FLX device have the same intended use but different shaped frames.
This approval expands the indications to include the use of these devices in patients who have non-valvular atrial fibrillation, are at increased risk for stroke, are recommended and suitable for blood thining medication (anticoagulation therapy), and have an appropriate reason to seek a non-drug alternative to anticoagulation therapy.
How does it work? The doctor inserts a flexible tube (delivery catheter) into the vein of the patient’s leg and advances the catheter to the upper right chamber of the heart (right atrium). A small hole is made through the wall between the two upper chambers of the heart (atrial septum) so that the catheter reaches the LAA. The doctor then pushes either the WATCHMAN or WATCHMAN FLX device into the LAA where it is opens up like an umbrella and is permantely implanted. Once the WATCHMAN or WATCHMAN FLX device is in place, a thin layer of tissue grows over it in about 45 days. This keeps blood clots in the LAA from entering the bloodstream.
When is it used? The WATCHMAN or WATCHMAN FLX device is intended to be used in patients who have atrial fibrillation (AFib) not related to heart valve disease (non-valvular atrial fibrillation). In AFib, the two upper chambers of the heart (right and left atria) no longer contract together in a coordinated manner and the heartbeat (pulse) becomes irregular. Because the right and left atria no longer contract normally in AFib, the blood flow in the heart can be slower than normal. This change in blood flow may cause blood clots to form. During Afib, most blood clots that develop in the heart develop in the LAA. If this occurs, these blood clots can break loose, travel through the bloodstream, and block a blood vessel in the brain and cause a stroke.
What will it accomplish? In a clinical study, in 395 out of 400 (96%) patients who had WATCHMAN FLX device implanted were able to stop taking their blood thinning medications after 45 days. In addition, over 92% of patients were able to stop taking blood thinning medications after one year following the implant procedure.
Due to the similarities in the device design, the WATCHMAN FLX device data described above is also applicable to the WATCHMAN device.
When should it not be used? The WATCHMAN or WATCHMAN FLX device should not be used in patients who:
- currently have a blood clot in their heart.
- have had surgical repair of the atrial septum (wall between the upper chambers of the heart), or have a device placed in the atrial septum.
- have a LAA that is too large or too small to fit the WATCHMAN or WATCHMAN FLX device.
- cannot have blood thinning medicines (anticoagulation therapy, P2Y12 inhibitors, and aspirin).
- have sensitivity to Nitinol (nickel or titanium) or any other material that is part of the device.
Additional information (including warnings, precautions, and adverse events):