Drug Trials Snapshots: AGAMREE
HOW TO USE THIS SNAPSHOT
The information provided in Snapshots highlights who participated in the key clinical trials that supported the original FDA approval of this drug, and whether there were differences among sex, race, age, and ethnic groups. The “MORE INFO” bar shows more detailed, technical content for each section. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of the drugs.
LIMITATIONS OF THIS SNAPSHOT:
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your healthcare provider about the benefits and risks of a drug.
Some of the information in this Snapshot is for presentation purposes and does not represent the approved conditions of use of this drug. Refer to the AGAMREE Prescribing Information for all of the approved conditions of use of this drug (e.g., indication(s), population(s), dosing regimen(s), safety information).
Snapshots are limited to the information available at the time of the original approval of the drug and do not provide information on who participated in clinical trials that supported later approvals for additional uses of the drug (if applicable).
AGAMREE (vamorolone)
ah gam' ree
Santhera Pharmaceuticals (Switzerland), LTD
Original Approval date: October 26, 2023
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
AGAMREE is a corticosteroid indicated for the treatment of Duchenne muscular dystrophy (DMD) in patients 2 years of age and older. DMD is an X-linked genetic disorder that leads to progressive muscle weakness and loss of muscle function, including loss of the ability to walk, problems with the muscles that control breathing and heart function, and death, usually by 30 years of age.
How is this drug used?
AGAMREE is taken by mouth once daily, preferably with a meal. The recommended dosage is 6 mg/kg of weight, up to a maximum daily dosage of 300 mg for patients weighing more than 50 kg. Some patients may respond to a dose of 2 mg/kg daily. Doses may be gradually reduced to 2 mg/kg/day as needed, based on individual tolerability.
Who participated in the clinical trials?
The FDA approved AGAMREE based on evidence from a single clinical trial of 121 male patients with DMD who were 4 to <7 years of age. The trial (Study 1) was conducted at 33 sites in 11 countries in Australia, Belgium, Canada, Czech Republic, Spain, United Kingdom, Greece, Israel, Netherlands, Sweden, and the United States.
In addition to Study 1, safety was also evaluated in a separate, open-label study of pediatric patients with DMD aged 2 to <4 years (N=16) and pediatric patients with DMD aged 7 to <18 years (N=16). Adverse reactions in these pediatric patients were similar to those seen in the Study 1 pediatric patients aged 4 to <7 years (N=121).
How were the trials designed?
AGAMREE was evaluated in one clinical trial of 121 patients with DMD.
How were the trials designed?
The effectiveness of AGAMREE for the treatment of DMD was evaluated in a multicenter, randomized, double-blind, parallel-group, placebo- and active-controlled, multinational 24-week study (Study 1). The study randomized 121 male patients with DMD to one of the following treatment groups: AGAMREE 6 mg/kg/day (N=30), AGAMREE 2 mg/kg/day (N=30), prednisone 0.75 mg/kg/day (N=31), or placebo (N=30) for 24 weeks. The safety population included 118 DMD patients who received study medication during the placebo-controlled part of the study. After 24 weeks, patients on prednisone and placebo received either AGAMREE 6 mg/kg/day (N=29) or AGAMREE 2 mg/kg/day (N=29) for an additional 20 weeks. The study included patients 4 to <7 years of age at the time of enrollment in the study who were corticosteroid naïve and ambulatory, with a confirmed diagnosis of DMD. At baseline, patients had a mean age of 5.4 years, 83% were White, 10% were Asian, and 96% were not Hispanic or Latino.
The primary endpoint was the change from baseline to Week 24 in Time to Stand Test (TTSTAND) velocity for AGAMREE 6 mg/kg/day compared to placebo. TTSTAND velocity is a measure of muscle function that measures the time required for the patient to stand to an erect position from a supine position (floor). The key secondary endpoints consisted of change from baseline to Week 24 in TTSTAND velocity (AGAMREE 2 mg/kg/day versus placebo), 6 Minute Walk Test (6MWT) distance (AGAMREE 6 mg/kg/day versus placebo and 2 mg/kg/day versus placebo), and Time to Run or Walk 10 meters (TTRW) velocity (AGAMREE 6 mg/kg/day versus placebo and 2 mg/kg/day versus placebo). The 6MWT measures the distance that a patient can walk on a flat, hard surface in a period of six minutes and TTRW measures the time that it takes a patient to run or walk 10 meters.
DEMOGRAPHICS SNAPSHOT
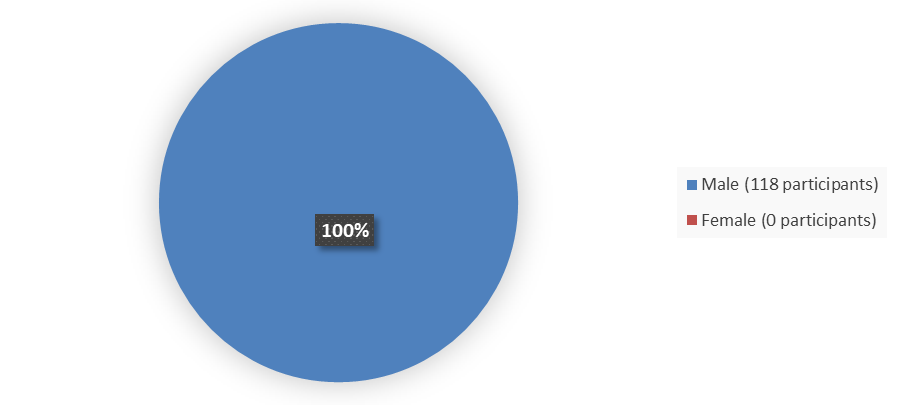
Figure 1 summarizes how many male and female patients were enrolled in the clinical trial used to evaluated efficacy of AGAMREE.
Figure 1. Baseline Demographics by Sex
Source: Adapted from FDA Review
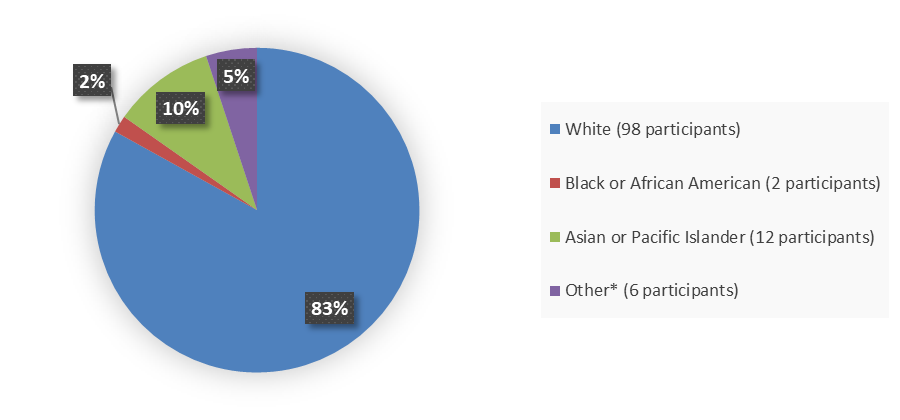
Figure 2 summarizes the percentage of patients by race enrolled in the clinical trial used to evaluate the efficacy of AGAMREE.
Figure 2. Baseline Demographics by Race
Source: Adapted from FDA Review
* Other includes American Indian or Alaska Native, multiple races, and unknown race.
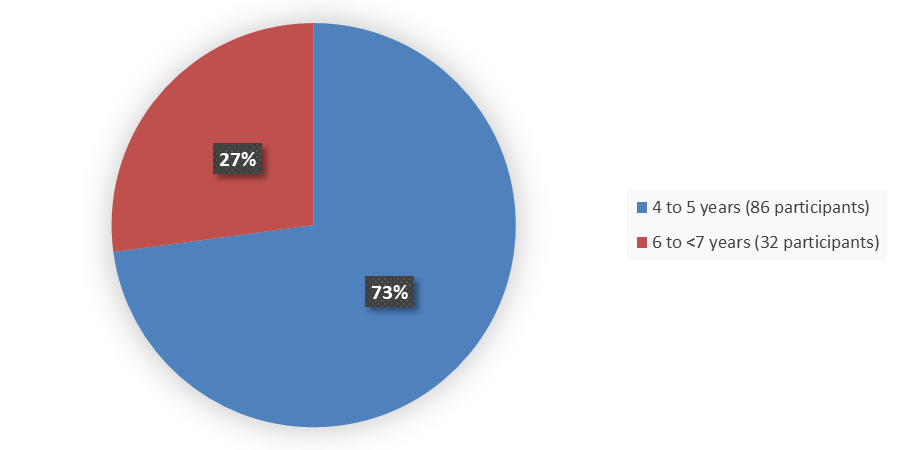
Figure 3 summarizes the percentage of patients by age enrolled in the clinical trial used to evaluate the efficacy of AGAMREE.
Figure 3. Baseline Demographics by Age
Source: Adapted from FDA Review
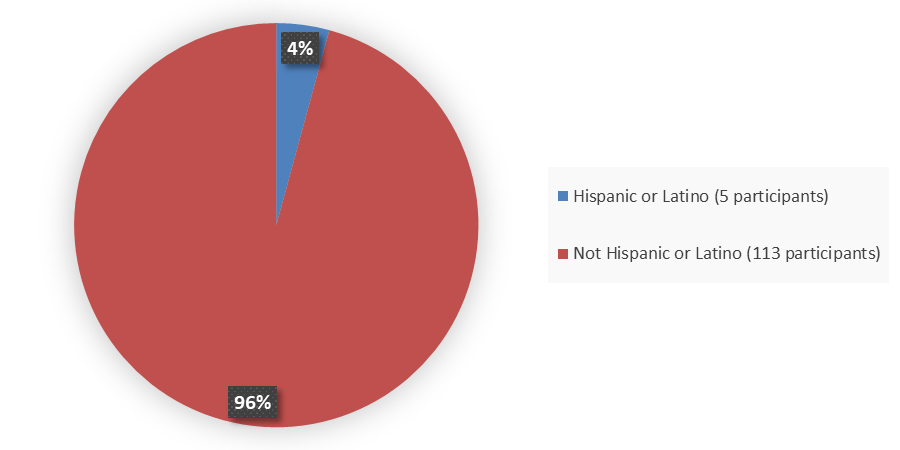
Figure 4 summarizes the percentage of patients by ethnicity enrolled in the clinical trial used to evaluate the efficacy of AGAMREE.
Figure 4. Baseline Demographics by Ethnicity
Source: Adapted from FDA Review
Who participated in the trials?
Table 1. Baseline Demographics of Efficacy Trial by Age, Race, Sex, Ethnicity, and Region
|
|
|
|
AGAMREE 2 mg/kg/day |
AGAMREE 6 mg/kg/day |
|
|---|---|---|---|---|---|
|
Sex |
|||||
|
Male |
29 |
31 |
30 |
28 |
118 |
|
Female |
0 |
0 |
0 |
0 |
0 |
|
Age, years |
|||||
|
Mean (SD) |
5.4 (0.83) |
5.5 (0.86) |
5.3 (0.91) |
5.4 (0.88) |
5.4 (0.86) |
|
Median |
5.3 |
5.3 |
5.2 |
5.5 |
5.3 |
|
IQR |
4.8, 6.0 |
4.9, 6.1 |
4.4, 6.2 |
4.5, 6.1 |
4.7, 6.1 |
|
Min, max |
4.1, 7.0 |
4.0, 7.0 |
4.1, 7.0 |
4.1, 6.8 |
4.0, 7.0 |
|
Age group, years, n (%) |
|||||
|
4 to 5 |
22 (75.9) |
22 (71.0) |
21 (70.0) |
21 (75.0) |
86 (72.9) |
|
6 to <7 |
7 (24.1) |
9 (29.0) |
9 (30.0) |
7 (25.0) |
32 (27.1) |
|
Ethnicity, n (%) |
|||||
|
Hispanic or Latino |
2 (6.9) |
0 |
2 (6.7) |
1 (3.6) |
5 (4.2) |
|
Not Hispanic or Latino |
27 (93.1) |
31 (100.0) |
28 (93.3) |
27 (96.4) |
113 (95.8) |
|
Race, n (%) |
|||||
|
American Indian of Alaska Native |
0 |
1 (3.2) |
0 |
0 |
1 (<1) |
|
Asian |
2 (6.9) |
3 (9.7) |
4 (13.3) |
3 (10.7) |
12 (10.2) |
|
Black or African American |
0 |
0 |
1 (3.3) |
1 (3.6) |
2 (1.7) |
|
White |
25 (86.2) |
25 (80.6) |
25 (83.3) |
23 (82.1) |
98 (83.1) |
|
Unknown |
1 (3.4) |
0 |
0 |
0 |
1 (<1) |
|
Multiple |
1 (3.4) |
2 (6.5) |
0 |
1 (3.6) |
4 (3.4) |
|
Region, n (%) |
|||||
|
Europe |
18 (62.1) |
19 (61.3) |
11 (36.7) |
18 (64.3) |
66 (55.9) |
|
United States |
11 (37.9) |
12 (38.7) |
19 (63.3) |
10 (35.7) |
52 (44.1) |
Source: Adapted from FDA Review
Abbreviations: IQR, interquartile range; SD, standard deviation
What are the benefits of this drug?
Clinical trial evidence from the single controlled study indicates that AGAMREE improves the ability of DMD patients to stand up, walk, and run.
What are the benefits of this drug (results of trials used to assess efficacy)?
The primary endpoint and key secondary endpoints were all met for the AGAMREE 6 mg/kg/day treatment group compared to placebo (TTSTAND, 6MWT, and TTRW). The AGAMREE 2 mg/kg/day treatment group had a statistically significant improvement compared to placebo for the primary endpoint, TTSTAND, and one of the key secondary endpoints, 6MWT, but was not statistically significant compared to placebo for TTRW.
In the 24-week double-blind, placebo-controlled study, the mean speed of standing up (TTSTAND, rises/second) increased in subjects who received AGAMREE 6 mg/kg but decreased in subjects who received placebo (p=0.0018). There was also a mean 29 meter improvement in the distance walked over time (6MWT) for subjects who received AGAMREE 6 mg/kg (p=0.0033) compared to a mean 24-meter decrease in the placebo group. Additionally, the speed of running or walking 10 meters increased in the AGAMREE 6 mg/kg group by a mean of 0.2 meters/second (0.4 miles/hour) compared to the placebo group (p=0.0018).
Table 2. Efficacy Results, Study 1: Change From Baseline to Week 24 on TTSTAND, 6MWT, and TTRW Compared to Placebo
|
|
AGAMREE |
AGAMREE |
|
|---|---|---|---|
|
TTSTAND velocity (rises/sec) |
|||
|
Baseline |
0.184 |
0.186 |
0.200 |
|
Mean change from baseline |
0.033 |
0.048 |
-0.012 |
|
Difference from placebo (95% CI) |
0.045 (0.008, 0.082) |
0.060 (0.023, 0.098) |
NA |
|
p-value |
0.017 |
0.002a |
NA |
|
6MWT distance (meters) |
|||
|
Baseline |
316 |
313 |
355 |
|
Mean change from baseline |
27 |
29 |
-14 |
|
Difference from placebo (95% CI) |
40 (13, 68) |
42 (16, 69) |
NA |
|
p-value |
0.004 |
0.002 |
NA |
|
TTRW velocity (meters/sec) |
|||
|
Baseline |
1.563 |
1.600 |
1.735 |
|
Mean change from baseline |
0.141 |
0.258 |
0.014 |
|
Difference from placebo (95% CI) |
0.127 (-0.026, 0.281) |
0.244 (0.093, 0.395) |
NA |
|
p-value |
0.103 |
0.002 |
NA |
Source: AGAMREE Prescribing Information
Baseline values are presented with descriptive statistics (mean). Mean changes and differences are model-based least-squares means and mean differences. Positive numbers indicate improvement as compared with the baseline value.
a Primary endpoint
Abbreviations: 6MWT, 6 Minute Walk Test; CI, confidence interval; NA, not applicable; TTRW, Time to Run or Walk 10 meters; TTSTAND, Time to Stand Test
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
- Sex: The study included only males because DMD is an X-linked recessive disease and female manifesting carriers are very rare.
- Race: AGAMREE worked similarly in subjects of different races, although the majority of subjects were White, which makes the results difficult to interpret.
- Age: AGAMREE worked similarly in patients younger and older than 5 years of age. The initial clinical trial was only in patients 4 to <7 years of age.
Were there any differences in how well the drug worked in clinical trials among sex, race, and age groups?
Table 3. Subgroup Analyses for Primary Endpoint: TTSTAND Velocity Change From Baseline to Week 24 (AGAMREE 6 mg/kg vs. Placebo)
|
|
AGAMREE |
Placebo |
|---|---|---|
|
Age group 1 |
||
|
≤5 years, n |
10 |
11 |
|
Mean at baseline (SD) |
0.173 (0.044) |
0.195 (0.049) |
|
LS mean difference from baseline (SE) |
0.0678 (0.0189) |
0.0196 (0.0174) |
|
LS mean difference from placebo (95% CI) |
0.0482 (-0.0038, 0.1002) |
|
|
>5 years, n |
18 |
17 |
|
Mean at baseline (SD) |
0.192 (0.066) |
0.204 (0.073) |
|
LS mean difference from baseline (SE) |
0.0417 (0.0172) |
-0.0251 (0.0177) |
|
LS mean difference from placebo (95% CI) |
0.0668 (0.0175, 0.1160) |
|
|
Age group 2 |
||
|
≤6 years, n |
21 |
21 |
|
Mean at baseline (SD) |
0.179 (0.049) |
0.193 (0.051) |
|
LS mean difference from baseline (SE) |
0.0632 (0.0153) |
0.0066 (0.0150) |
|
LS mean difference from placebo (95% CI) |
0.0565 (0.0139, 0.0991) |
|
|
>6 years, n |
7 |
7 |
|
Mean at baseline (SD) |
0.205 (0.084) |
0.221 (0.097) |
|
LS mean difference from baseline (SE) |
0.0225 (0.0222) |
-0.0532 (0.0222) |
|
LS mean difference from placebo (95% CI) |
0.0757 (0.0107, 0.1407) |
|
|
Race |
||
|
White, n |
23 |
24 |
|
Mean at baseline (SD) |
0.185 (0.051) |
0.197 (0.067) |
|
LS mean difference from baseline (SE) |
0.0316 (0.0144) |
-0.0187 (0.0140) |
|
LS mean difference from placebo (95% CI) |
0.0503 (0.0110, 0.0897) |
|
|
Other, n |
5 |
4 |
|
Mean at baseline (SD) |
0.189 (0.095) |
0.221 (0.044) |
|
LS mean difference from baseline (SE) |
0.1330 (0.0355) |
0.0335 (0.0434) |
|
LS mean difference from placebo (95% CI) |
0.0995 (-0.005, 0.2039) |
|
|
Region |
||
|
United States, n |
10 |
10 |
|
Mean at baseline (SD) |
0.171 (0.041) |
0.204 (0.068) |
|
LS mean difference from baseline (SE) |
0.0316 (0.0217) |
-0.0138 (0.0214) |
|
LS mean difference from placebo (95% CI) |
0.0455 (-0.0154, 0.1063) |
|
|
Europe, n |
18 |
18 |
|
Mean at baseline (SD) |
0.193 (0.067) |
0.198 (0.064) |
|
LS mean difference from baseline (SE) |
0.0566 (0.0180) |
-0.0121 (0.0177) |
|
LS mean difference from placebo (95% CI) |
0.0687 (0.0194, 0.1180) |
|
Source: Adapted from FDA Review
Abbreviations: CI, confidence interval; LS, least-squares; SD, standard deviation; SE, standard error
What are the possible side effects?
The most common adverse reactions (>10% for AGAMREE and greater than placebo) are cushingoid features, psychiatric disorders, vomiting, weight increased, and vitamin D deficiency.
What are the possible side effects (results of trials used to assess safety)?
- Alterations in Endocrine Function: Hypothalamic-pituitary-adrenal axis suppression, cushingoid features, and hyperglycemia can occur. Monitor patients for these conditions with chronic use of AGAMREE.
- Immunosuppression and Increased Risk of Infection: Increased risk of new infections, exacerbation, dissemination, or reactivation of latent infections, which can be severe and at times fatal; signs and symptoms of infections may be masked.
- Alterations in Cardiovascular/Renal Function: Monitor for elevated blood pressure and monitor sodium and potassium levels in patients chronically treated with AGAMREE.
- Gastrointestinal Perforation: Increased risk in patients with certain GI disorders; signs and symptoms may be masked.
- Behavioral and Mood Disturbances: May include euphoria, insomnia, mood swings, personality changes, severe depression, and psychosis.
- Effects on Bones: Monitor for decreases in bone mineral density with chronic use of AGAMREE.
- Ophthalmic Effects: May include cataracts, infections, and glaucoma; monitor intraocular pressure in patients chronically treated with AGAMREE.
- Vaccination: Do not administer live or live attenuated vaccines to patients receiving immunosuppressive doses of corticosteroids. Administer live attenuated or live vaccines at least 4 to 6 weeks prior to starting AGAMREE.
Table 4. Adverse Reactions in Patients With DMD That Occurred in ≥5% of Patients Treated With AGAMREE and More Frequently Than in Patients Who Received Placebo During 24 Weeks (Study 1)
|
Adverse Reaction |
AGAMREE |
AGAMREE |
|
|---|---|---|---|
|
Cushingoid features |
7 |
29 |
0 |
|
Psychiatric disordersa |
7 |
21 |
14 |
|
Vomiting |
17 |
14 |
7 |
|
Weight increased |
0 |
11 |
3 |
|
Vitamin D deficiency |
7 |
11 |
0 |
|
Cough |
10 |
7 |
3 |
|
Headache |
7 |
7 |
3 |
|
Diarrhea |
3 |
7 |
3 |
|
Increased appetite |
3 |
7 |
3 |
|
Rhinitis |
3 |
7 |
3 |
Source: AGAMREE Prescribing Information
a Includes the following adverse reactions that occurred more frequently in the AGAMREE group than in placebo: abnormal behavior, aggression, agitation, anxiety, irritability, mood altered, sleep disorder, and stereotypy.
Abbreviations: DMD, Duchenne muscular dystrophy
Were there any differences in side effects of the clinical trials among sex, race, and age?
- Sex: The study included only males because DMD is an X-linked recessive disease and female manifesting carriers are very rare.
- Race: The occurrence of side effects was similar in subjects of different races, although the majority of subjects were White, which makes the result difficult to interpret.
- Age: The occurrence of side effects was similar in patients younger and older than 5 years of age.
Were there any differences in side effects of the clinical trials among sex, race, and age groups?
Table 5. Side Effects by Sex, Race, Age, and Ethnicity, Study 1
|
Characteristic |
Prednisone |
Placebo |
AGAMREE 2.0 mg/kg/day |
AGAMREE 6.0 mg/kg/day |
AGAMREE 6.0 mg/kg/day vs. Placebo |
|---|---|---|---|---|---|
|
Sex |
|||||
|
Male |
26/31 (83.9) |
23/29 (79.3) |
25/30 (83.3) |
25/28 (89.3) |
10.0 (-8.7, 28.6) |
|
Age group, years |
|||||
|
<5 |
8/8 (100) |
8/12 (66.7) |
13/14 (92.9) |
9/10 (90.0) |
23.3 (-9.2, 55.8) |
|
≥5 |
18/23 (78.3) |
15/17 (88.2) |
12/16 (75.0) |
16/18 (88.9) |
0.7 (-20.4, 21.8) |
|
Race |
|||||
|
American Indian or Alaska Native |
1/1 (100) |
0/0 (NA) |
0/0 (NA) |
0/0 (NA) |
NA |
|
Asian |
3/3 (100) |
1/2 (50.0) |
3/4 (75.0) |
3/3 (100) |
50.0 (-19.3, 119.3) |
|
Black or African American |
0/0 (NA) |
0/0 (NA) |
1/1 (100) |
1/1 (100) |
NA |
|
Multiple |
2/2 (100) |
1/1 (100) |
0/0 (NA) |
1/1 (100) |
0 (0, 0) |
|
Unknown |
0/0 (NA) |
1/1 (100) |
0/0 (NA) |
0/0 (NA) |
NA |
|
White |
20/25 (80.0) |
20/25 (80.0) |
21/25 (84.0) |
20/23 (87.0) |
7.0 (-13.9, 27.8) |
|
Ethnicity |
|||||
|
Hispanic or Latino |
0/0 (NA) |
2/2 (100) |
1/2 (50.0) |
1/1 (100) |
0 (0, 0) |
|
Not Hispanic or Latino |
26/31 (83.9) |
21/27 (77.8) |
24/28 (85.7) |
24/27 (88.9) |
11.1 (-8.5, 30.8) |
|
Is in United States |
|||||
|
United States |
7/8 (87.5) |
4/5 (80.0) |
6/10 (60.0) |
5/6 (83.3) |
3.3 (-42.7, 49.4) |
|
Non-United States |
19/23 (82.6) |
19/24 (79.2) |
19/20 (95.0) |
20/22 (90.9) |
11.7 (-8.5, 31.9) |
Source: Adapted from FDA Review
Abbreviations: CI, confidence interval; N, number of patients in treatment arm; n, number of patients with side effects; NA, not applicable; Ns, total number of patients for each specific subgroup and were assigned to that specific arm
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.