Drug Trials Snapshots: REZLIDHIA
HOW TO USE THIS SNAPSHOT
The information provided in Snapshots highlights who participated in the key clinical trials that supported the original FDA approval of this drug, and whether there were differences among sex, race, age, and ethnic groups. The “MORE INFO” bar shows more detailed, technical content for each section. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of the drugs.
LIMITATIONS OF THIS SNAPSHOT:
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your healthcare provider about the benefits and risks of a drug.
Some of the information in this Snapshot is for presentation purposes and does not represent the approved conditions of use of this drug. Refer to the REZLIDHIA Prescribing Information for all of the approved conditions of use of this drug (e.g., indication(s), population(s), dosing regimen(s), safety information).
Snapshots are limited to the information available at the time of the original approval of the drug and do not provide information on who participated in clinical trials that supported later approvals for additional uses of the drug (if applicable).
REZLIDHIA (olutasidenib)
REZ-LID-EE-AH
Rigel Pharmaceuticals, Inc
Original Approval date: 12/1/2022
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
REZLIDHIA is a prescription medicine used to treat adults with acute myeloid leukemia (AML) with an isocitrate dehydrogenase-1 (IDH1) mutation when the disease has come back (relapsed) or has not improved after one or more treatments (refractory). Your healthcare provider will perform a test to make sure that REZLIDHIA is right for you.
How is this drug used?
The recommended dose of REZLIDHIA is 150 mg taken by mouth twice daily (about 12 hours apart) around the same times each day. REZLIDHIA should be taken on an empty stomach at least 1 hour before or 2 hours after a meal.
Who participated in the clinical trials?
The FDA approved REZLIDHIA based on evidence from one clinical trial (NCT02719574) of 153 adult patients with AML whose disease has come back or has not improved with previous treatments. A total of 147 patients had a certain type of mutation called IDH1, which was confirmed using an FDA-approved test. The trial was conducted in the United States, Australia, Canada, France, Germany, Italy, Republic of Korea, Spain, and the United Kingdom.
How were the trials designed?
There was one trial that evaluated the benefit and side effects of REZLIDHIA in adult patients with AML whose disease has come or has not improved after previous treatments. All patients had a certain type of mutation called IDH1, which was confirmed with an FDA-approved test.
Patients received REZLIDHIA 150 mg by mouth twice daily. Treatment continued until either disease worsened or patients experienced unacceptable side effects.
The benefit of REZLIDHIA was evaluated by measuring how many patients had a complete remission (no evidence of disease) with full or partial recovery of blood counts after treatment, how long those patients remained in remission, and the percentage of patients who no longer required transfusions after treatment.
How were the trials designed?
The safety and efficacy of REZLIDHIA was established in one open-label, single-arm, multicenter clinical trial in 153 adult patients with relapsed or refractory AML and an IDH1 mutation. REZLIDHIA was given orally at a dose of 150 mg twice daily until disease progression, development of unacceptable side effects, or hematopoietic stem cell transplantation.
Efficacy was established based on the rate of rate of complete remission (CR) plus complete remission with partial hematologic recovery (CRh), the duration of CR+CRh, and the rate of conversion from transfusion dependence to transfusion independence.
DEMOGRAPHICS SNAPSHOT
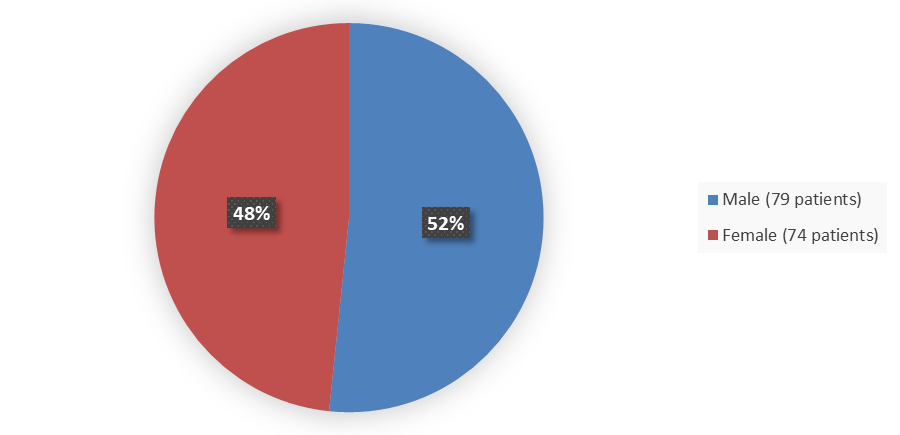
Figure 2. Demographics by Sex
Source: Adapted from FDA Review
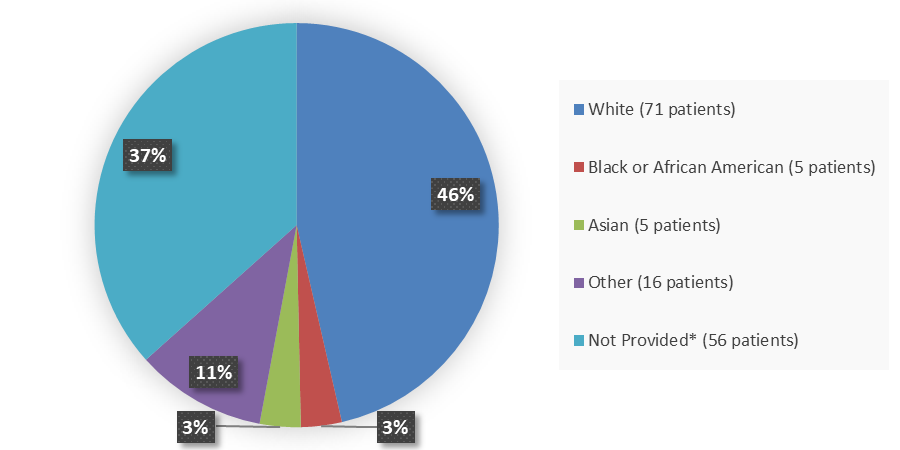
Figure 3. Demographics by Race
Source: Adapted from FDA Review
* Collection of race data not allowed in certain European nations per country specific regulations.
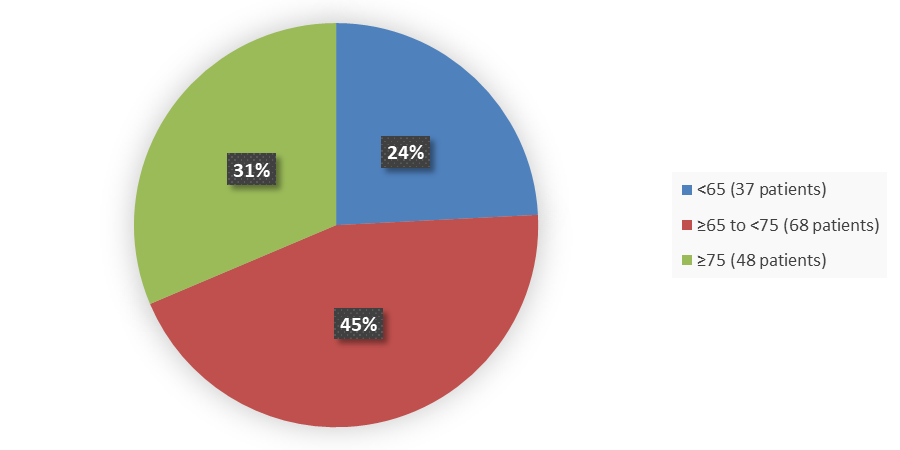
Figure 4. Baseline Demographics by Age
Source: Adapted from FDA Review
Who participated in the trials?
Table 5 summarizes demographics of all patients enrolled in the clinical trial who received REZLIDHIA at the recommended dose (safety population).
Table 5. Baseline Demographics of Patients Enrolled in the Clinical Trial (Safety Population)
|
Demographic Characteristic |
REZLIDHIA |
|---|---|
|
Sex |
|
|
Male |
79 (52) |
|
Female |
74 (48) |
|
Race |
|
|
White |
71 (46) |
|
Black |
5 (3) |
|
Asian |
5 (3) |
|
Other |
16 (11) |
|
Not provided or unknown* |
56 (37) |
|
Age, years |
|
|
Mean |
68.7 |
|
Median |
71.0 |
|
Min, max |
32, 89 |
|
Age group, years |
|
|
18 to <65 |
37 (24) |
|
≥65 to <75 |
68 (44) |
|
≥75 |
48 (31) |
|
Ethnicity |
|
|
Not Hispanic or Latino |
Not reported |
|
Hispanic or Latino |
Not reported |
|
Not provided* |
153 (100) |
|
Trial site |
|
|
North America |
29 (19) |
|
European Union |
104 (68) |
|
Asia-Pacific |
20 (13) |
Source: Adapted from FDA Review
* Collection of data not allowed by local authority
What are the benefits of this drug?
In a clinical trial with 147 adult patients with relapsed or refractory AML with an IDH1 mutation, 35% of patients had no evidence of disease and full recovery of blood counts (complete remission) or no evidence of disease and partial recovery of blood counts after treatment. The time to this response was about 2 months, and the response lasted about 26 months.
Of the 86 patients who needed blood and/or platelet transfusions before treatment with REZLIDHIA, 34% no longer needed transfusions for at least 56 days during treatment.
Of the 61 patients who did not need transfusions of both blood and platelets before treatment with REZLIDHIA, 64% went at least 56 days without needing a transfusion during treatment.
What are the benefits of this drug (results of trials used to assess efficacy)?
Efficacy was established based on the rate of CR+CRh, the duration of CR+CRh, and the rate of conversion from transfusion dependence to transfusion independence. The efficacy results are shown in Table 1.
Table 1. Efficacy Results in Patients With Relapsed or Refractory AML
|
Endpoint |
REZLIDHIA |
|
|---|---|---|
|
N=147 |
95% CI |
|
|
CR+CRh1,2, n (%) |
51 (35) |
27, 43 |
|
Median DOR, months3 |
25.9 |
13.5, NR |
|
CR1, n (%) |
47 (32) |
25, 40 |
|
Median DOR3, months |
28.1 |
13.8, NR |
|
CRh2, n (%) |
4 (2.7) |
0.7, 6.8 |
|
Observed DOCRh3, months |
1.8, 5.6, 13.5, 28.5+ |
|
Source: REZLIDHIA Prescribing Information
1 CR was defined as <5% blasts in the bone marrow, no blasts with Auer rods, no disease outside the bone marrow, and full recovery of blood counts (platelets >100,000/microliter and absolute neutrophil counts [ANC] >1,000/microliter); CRh was defined as <5% blasts in the bone marrow, no evidence of disease, and partial recovery of peripheral blood counts (platelets >50,000/microliter and ANC >500/microliter).
2 CR+CRh rate was consistent across all baseline demographic and baseline disease characteristic subgroups with the exception of IDH1 R132H mutation (CR+CRh 17%).
3 Duration of response was defined as the time since first response of CR or CRh to the date of relapse or death, whichever is earlier. Abbreviations: AML, acute myeloid leukemia; CI, confidence interval; CR, complete remission; CRh, complete remission with partial hematologic recovery; DOR, duration of response; DOCRh, duration of complete remission with partial hematologic recovery; NR, not reached
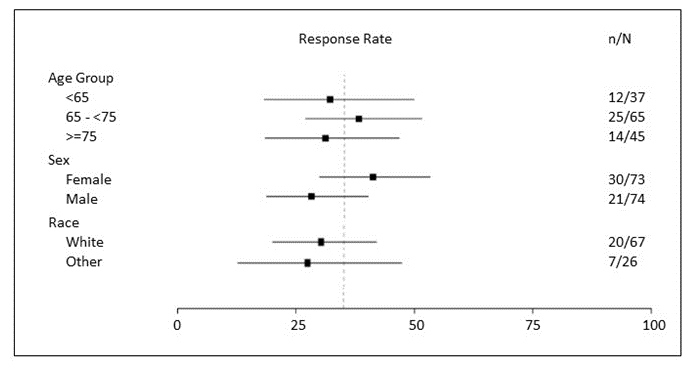
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
- Sex: REZLIDHIA worked similarly in males and females.
- Race: The number of patients of races other than White was small; therefore, differences in how well the drug worked among races could not be determined.
- Age: REZLIDHIA worked similarly in patients younger and older than 65 years of age.
Were there any differences in how well the drug worked in clinical trials among sex, race, and age groups?
Figure 1 summarizes exploratory efficacy results by demographic subgroups.
Figure 1. Graph of Response Rate (CR+CRh) by Age, Sex, and Race
Source: Adapted from FDA review
Abbreviations: CR, complete remission; CRh, complete remission with partial hematologic recovery
What are the possible side effects?
REZLIDHIA may cause a serious and life-threatening side effect called differentiation syndrome. Another serious side effect is liver toxicity.
The most common side effects of REZLIDHIA are abnormal liver function tests, nausea, feeling tired or unwell, joint pain, constipation, shortness of breath, fever, rash, mouth sores, diarrhea, and changes in certain blood tests.
What are the possible side effects (results of trials used to assess safety)?
Table 2 summarizes the most common side effects in patients with relapsed or refractory AML in the clinical trial and Table 3 summarizes the most common laboratory abnormalities.
Table 2. Side Effects Reported in ≥10% (Any Grade) of Patients With Relapsed or Refractory AML
|
Body System |
REZLIDHIA |
|
|---|---|---|
|
All Grades |
Grade 3 or 4 |
|
|
Gastrointestinal disorders |
||
|
Nausea |
38 |
0 |
|
Constipation |
26 |
0 |
|
Mucositis2 |
23 |
3 |
|
Diarrhea |
20 |
1 |
|
Abdominal pain1 |
18 |
1 |
|
Vomiting |
17 |
1 |
|
General disorders and administration site conditions |
||
|
Fatigue/malaise1 |
36 |
3 |
|
Pyrexia |
24 |
1 |
|
Edema1 |
18 |
3 |
|
Musculoskeletal and connective tissue disorders |
||
|
Arthralgia3 |
28 |
3 |
|
Blood system and lymphatic disorders |
||
|
Leukocytosis |
25 |
9 |
|
Differentiation syndrome*,4 |
16 |
8 |
|
Respiratory, thoracic and mediastinal disorders |
||
|
Dyspnea*,5 |
24 |
5 |
|
Cough1 |
17 |
1 |
|
Skin and subcutaneous tissue disorders |
||
|
Rash1 |
24 |
1 |
|
Investigations |
||
|
Transaminitis6 |
20 |
12 |
|
Metabolism and nutrition disorders |
||
|
Decreased appetite |
16 |
2 |
|
Nervous system disorders |
||
|
Headache |
13 |
0 |
|
Vascular disorders |
||
|
Hypertension1 |
10 |
5 |
Source: REZLIDHIA Prescribing Information
* Includes fatal adverse reaction
1 Includes multiple similar adverse reaction terms
2 Mucositis includes gingival hypertrophy, gingivitis, gingivitis ulcerative, oral disorder, colitis, mouth ulceration, stomatitis, tongue ulceration, oral pain, oropharyngeal pain, pharyngitis, proctalgia, and colitis ischemic
3 Arthralgia grouped term includes arthralgia, bone pain, back pain, neck pain, pain in extremity, arthritis, joint effusion, joint range of motion decreased, and joint swelling
4 Symptoms of differentiation syndrome in patients treated with REZLIDHIA included leukocytosis, dyspnea, pulmonary infiltrates/ pleuropericardial effusion, kidney injury, fever, edema, pyrexia, and weight gain
5 Dyspnea grouped term includes acute respiratory distress syndrome, dyspnea, dyspnea exertional, hypoxia, oxygen saturation decreased, respiratory distress, and respiratory failure
6 Transaminitis grouped term includes alanine aminotransferase increased, aspartate aminotransferase increased, hepatic enzyme increased, hypertransaminasemia, liver function test abnormal, liver function test increased, transaminases increased, hepatitis acute, and blood alkaline phosphatase increased
Abbreviations: AML, acute myeloid leukemia
Table 3. Most Common (≥10%) New or Worsening Laboratory Abnormalities Reported in Patients With Relapsed or Refractory AML
|
|
REZLIDHIA1 |
|
|---|---|---|
|
All Grades |
Grade 3 or 4 |
|
|
Aspartate aminotransferase increased |
47 |
10 |
|
Alanine aminotransferase increased |
46 |
13 |
|
Potassium decreased |
46 |
9 |
|
Sodium decreased |
42 |
7 |
|
Alkaline phosphatase increased |
42 |
7 |
|
Creatinine increased |
38 |
2 |
|
Lymphocytes increased |
26 |
3 |
|
Bilirubin increased |
26 |
2 |
|
Uric acid increased |
25 |
3 |
|
Lipase increased |
24 |
8 |
Source: REZLIDHIA Prescribing Information
1 The denominator used to calculate the rate varied from 143 to 152 based on the number of patients with a baseline value and at least one post-treatment value
Abbreviations: AML, acute myeloid leukemia
Were there any differences in side effects of the clinical trials among sex, race, and age?
- Sex: The occurrence of side effects was similar in males and females.
- Race: The number of patients with known races other than White was small; therefore, meaningful differences in the occurrence of side effects among races could not be determined.
- Age: The occurrence of side effects was similar in patients younger and older than 65 years of age.
Were there any differences in side effects of the clinical trials among sex, race, and age groups?
Table 4 summarizes grade 3 and 4 side effects in the clinical trial by sex, race, and age subgroup.
Table 4. Subgroup Analyses of Side Effects
|
REZLIDHIA |
|||
|---|---|---|---|
|
|
All Patients |
All Grades |
Grades 3 to 4 |
|
Sex, n (%) |
|||
|
Female |
74 (48.4) |
74/74 (100) |
63/74 (85.1) |
|
Male |
79 (51.6) |
79/79 (100) |
66/79 (83.5) |
|
Race, n (%) |
|||
|
White |
71 (46.4) |
71/71 (100) |
63/71 (88.7) |
|
Asian |
5 (3.3) |
5/5 (100) |
4/5 (80.0) |
|
Black or African American |
5 (3.3) |
5/5 (100) |
4/5 (80.0) |
|
Other |
16 (10.5) |
16/16 (100) |
15/16 (93.8) |
|
Missing |
56 (36.6) |
56/56 (100) |
43/56 (76.8) |
|
Age group, years, n (%) |
|||
|
<65 years |
37 (24.2) |
37/37 (100) |
29/37 (78.4) |
|
≥65 years |
116 (75.8) |
116/116 (100) |
100/116 (86.2) |
Source: Adapted from FDA Review
Abbreviations: TEAE, treatment-emergent adverse event
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.