Infant Formula Resources for Parents and Caregivers
There’s nothing as important to families as the health and safety of their babies. The U.S. Food and Drug Administration and other federal partners are working to expand and diversify the infant formula market, so you have more options and a more stable supply.
Below you can find important information about new formulas in the U.S. market and how to ensure you are safely purchasing and preparing formulas for your baby.
Imported Infant Formula
Having a diversity of infant formula suppliers is key to making sure you can find the type of formula you need for your baby.
In 2022, the FDA helped address supply issues through its enforcement discretion policy, under which certain infant formulas that the FDA had reviewed for information relating to safety and nutritional adequacy entered the U.S. market on a temporary basis. Many of the companies supplying those products have expressed an interest in staying in the U.S. market permanently.
You can learn more about imported regular formulas and imported specialty formulas for infants with special medical needs by going through the tables on the FDA’s enforcement discretion webpage. You can see the product labels by clicking on the product name.
It is important to talk with your child’s health care provider for advice and the appropriate formulas to use as you consider your options.
Safely Preparing Imported Formula
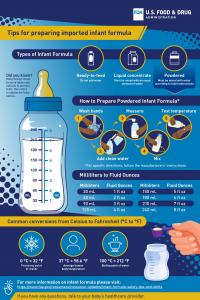
The labels and directions on imported formulas may have terms you are not used to seeing, such as metric measurements. To help you safely prepare imported infant formula, the FDA created a tip sheet. It shows conversion measurements from milliliters to fluid ounces and temperatures from Celsius to Fahrenheit (◦C to ◦F).
Video Gallery
Learn the answers to frequently asked questions about imported infant formula, including questions about safety measures, how to prepare powdered formula and more.
Safety Review Process
Watch: Learn about what the FDA is doing to ensure the safety of imported infant formula.
Infant formula that is being imported to the U.S. in accordance with FDA’s enforcement discretion policy undergoes a thorough review by the FDA. FDA’s review of the information provided by the companies in their enforcement discretion requests included looking at the:
- Ingredients
- Nutrient testing
- Manufacturing safety
- Allergen labeling
- Directions for preparation
Tips on Where to Find Products and Comparable Formulas
Although the supply of infant formula is steadily increasing, you may follow these tips to help find safe substitutes in the interim, including trying a new brand of formula (see list of comparable formulas), talking to a pediatrician or health care provider about submitting an urgent request for specialized formula, or contacting infant formula companies directly, may be able to help you find formula or safe substitutes.
Questions and Answers on Infant Formula
The FDA has developed a series of questions and answers regarding the nutritional quality and safety of infant formula that parents and caregivers may find useful: Infant Formula Q&A.
Do Not Make Homemade Infant Formula
The FDA advises parents and caregivers to not make or feed homemade infant formula to infants. Homemade infant formula recipes have not been evaluated by the FDA and may lack nutrients vital to an infant’s growth.
- Infant Formula: Safety Do's and Don'ts
- FDA Advises Parents and Caregivers to Not Make or Feed Homemade Infant Formula to Infants
Avoiding Counterfeit Infant Formula
The FDA monitors online marketplaces for counterfeit products. When such products are found, the FDA works with major online retailers to remove those products from sale on online sites. Additionally, since many such products originate overseas, the FDA targets and examines these products at ports of entry. The FDA also monitors and follows up on information that comes from outside the agency, such as consumer complaints about potential counterfeit products.
The FDA has information on what counterfeit formulas are and how you can avoid buying them. And the Federal Trade Commission has information to help you avoid scammers online.
Preventing Cronobacter Illness
Cronobacter contamination and infections are rare. It is still important to know the risks and what you can do to minimize them when preparing powdered infant formula.
- What to Know About the Risk of Cronobacter in Powdered Infant Formula
- Help Prevent Cronobacter Illness: Prepare and Store Powdered Infant Formula Safely
- CDC Information on Cronobacter Infection and Infants