Drug Trials Snapshot: FOTIVDA
HOW TO USE THIS SNAPSHOT
The information provided in Snapshots highlights who participated in the key clinical trials that supported the original FDA approval of this drug, and whether there were differences among sex, race, age, and ethnic groups. The “MORE INFO” bar shows more detailed, technical content for each section. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of the drugs.
LIMITATIONS OF THIS SNAPSHOT:
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your healthcare provider about the benefits and risks of a drug.
Some of the information in this Snapshot is for presentation purposes and does not represent the approved conditions of use of this drug. Refer to the FOTIVDA Prescribing Information for all of the approved conditions of use of this drug (e.g., indication(s), population(s), dosing regimen(s), safety information).
Snapshots are limited to the information available at the time of the original approval of the drug and do not provide information on who participated in clinical trials that supported later approvals for additional uses of the drug (if applicable).
FOTIVDA (tivozanib)
(foh-tiv-dah)
Aveo Pharmaceuticals, Inc.
Approval date: March 10, 2021
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
FOTIVDA is a prescription medicine used to treat adult patients with advance kidney cancer (advanced renal cell carcinoma or RCC) that has been treated with 2 or more prior medicines and has come back or did not respond to treatment.
How is this drug used?
Take FOTIVDA 1 time each day for 21 days on treatment, followed by 7 days off treatment. This is 1 cycle of treatment (28 days). You will repeat this cycle for as long as your healthcare provider tells you to.
Who participated in the clinical trials?
The FDA approved FOTIVDA based on evidence from one clinical trial of 350 adult patients with advanced kidney cancer (renal cell carcinoma or RCC). The trials was conducted at 120 of sites in 12 of countries in North America and Europe. The number of patients representing efficacy findings may differ from the number of patients representing safety findings due to different pools of study participants analyzed for efficacy and safety.
What are the benefits of this drug?
Patients who received FOTIVDA lived longer without cancer progression in comparison to patients who received sorafenib.
What are the benefits of this drug (results of trials used to assess efficacy)?
Table 1 shows efficacy results. The primary endpoint (i.e. outcome measure) was progression free survival (PFS) for patients treated with FOTIVDA compared to patients treated with sorafenib. This was assessed by radiologists who were “blinded”, which means that they did not know patients’ assigned treatment. Overall survival and objective response rate (percent of patients who had tumor shrinkage by at least 30%) were secondary endpoints.
Table 1: Efficacy Results
|
Endpoint |
FOTIVDA |
Sorafenib |
|---|---|---|
|
Progression Free Survival (PFS)* |
||
|
Events, n (%) |
123 (70) |
123 (70) |
|
Progressive Disease |
103 (59) |
109 (62) |
|
Death |
20 (11) |
14 (8) |
|
Median (95% CI), months |
5.6 (4.8, 7.30 |
3.9 (3.7, 5.6) |
|
HR (95% CI)† |
0.73 (0.56, 0.95) |
|
|
P-value†† |
0.016 |
|
|
Overall Survival |
||
|
Deaths, n (%) |
125 (71) |
126 (72) |
|
Median (95% CI), months |
16.4 (13.4, 21.9) |
19.2 (14.9, 24.2) |
|
HR (95% CI)† |
0.97 (0.75, 1.24) |
|
|
Objective Response Rate % (95% CI)* |
18 |
8 |
|
Median duration of response in months (95% CI) |
NE |
5.7 |
Source: FOTIVDA Prescribing Information
CI: Confidence interval; HR: Hazard ratio (FOTIVDA/sorafenib); NE: not estimable
* Assessed by blinded independent radiology review committee according to RECIST v1.1.
† Based on the Cox proportional hazards model stratified by IMDC prognostic score and prior therapy.
†† Based on the log-rank test stratified by IMDC prognostic score and prior therapy.
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
- Sex: FOTIVDA worked similarly in males and females
- Race: The number of patients of races other than White was small. Therefore, differences among races in how well the FOTIVDA worked could not be determined.
- Age: FOTIVDA worked similarly in patients below and above 65 years of age.
Were there any differences in how well the drug worked in clinical trials among sex, race, and age groups?
Table 2. Subgroup Analyses of PFS
|
|
Number of Events/Total |
Hazard Ratio |
|
|---|---|---|---|
|
|
FOTIVDA |
Sorafenib |
|
|
Overall |
123/175 |
123/175 |
0.73 (0.56, 0.95) |
|
Sex |
|||
|
Male |
87/126 |
93/128 |
0.64 (0.48, 0.86) |
|
Female |
36/49 |
30/47 |
0.72 (0.44, 1.19) |
|
Age Category |
|||
|
<65 years |
69/98 |
68/95 |
0.75 (0.53, 1.05) |
|
≥65 years |
54/77 |
55/80 |
0.59 (0.40, 0.87) |
|
Race Category |
|||
|
White |
116/165 |
118/167 |
0.67 (0.51, 0.87) |
|
Other* |
7/10 |
5/8 |
0.75 (0.23, 2.48) |
Source: FDA Review
*18 participants were reported as race other than White
What are the possible side effects?
The most common side effects are fatigue, high blood pressure, diarrhea, decreased appetite, nausea, changes in voice, low thyroid hormone, cough, and pain in the mouth. Serious side effects that may occur with FOTIVDA treatment include high blood pressure, heart problems, blood clots, and bleeding. Other possible serious side effects that may occur with FOTIVDA treatment include protein in the urine and thyroid abnormalities.
Were there any differences in side effects of the clinical trials among sex, race, and age?
- Sex: Males had slightly fewer side effects on FOTIVDA than sorafenib. Women had slightly more side effects on FOTIVDA than sorafenib.
- Race: The occurrence of side effects in African American patients could not be determined due to the small number of African American patients. The occurrence of side effects was similar in White and Asian patients; however, Asian patients experienced skin reactions two to three times more frequently than White patients. (See Tables 5 and 6)
- Age: Patients below 65 years of age had fewer side effects than patients 65 years of age or older among all FOTIVDA trials where patients with RCC participated.
Were there any differences in side effects of the clinical trials among sex, race, and age groups?
Table 3: Safety Results in TIVO-3 by Sex
|
|
Men |
Women |
||
|---|---|---|---|---|
|
|
FOTIVDA |
Sorafenib |
FOTIVDA |
Sorafenib |
|
All grades |
124 (99%) |
92 (100%) |
47 (98%) |
47 (100%) |
|
Grade 3 or 4 |
81 (65%) |
93 (76%) |
34 (71%) |
30 (64%) |
|
Grade 5 |
12 (9.6%) |
10 (8.1%) |
5 (10%) |
4 (8.5%) |
Source: FDA Review
Table 4: Safety of FOTIVDA in Older Versus Younger Patients (Pooled Safety Population from TIVO-3 and 5 monotherapy clinical trials)
|
|
Tivozanib (n=1008) |
|
|---|---|---|
|
|
< 65 years old |
≥ 65 years old |
|
Any TEAE1 |
631 (89%) |
285 (96%) |
|
Grade 3-4 |
348 (49%) |
173 (58%) |
|
Grade 5 |
56 (7.9%) |
125 (8.4%) |
1 Treatment Emergent Adverse Event
Source: FDA Review
Table 5: Safety of FOTIVDA in White Versus Asian Patients (Pooled Safety Population from TIVO-3 and 5 monotherapy clinical trials)
|
|
White |
Asian |
|---|---|---|
|
All grades |
861 (91%) |
37 (95%) |
|
Grade 3 or 4 |
486 (51%) |
20 (51%) |
|
Grade 5 |
80 (8%) |
2 (5%) |
Source: FDA Review
Table 6: Skin Reactions in White Versus Asian Patients Taking FOTIVDA (Pooled Safety Population from TIVO-3 and 5 monotherapy clinical trials)
|
|
White |
Asian |
||
|---|---|---|---|---|
|
|
All grades |
Grade 3 or 4 |
All grades |
Grade 3 or 4 |
|
Rash |
134 (14%) |
6 (0.6%) |
15 (38%) |
3 (7.7%) |
|
Hand-foot skin reaction |
110 (12%) |
11 (1.1%) |
8 (21%) |
3 (7.7%) |
Source: FDA Review
DEMOGRAPHICS SNAPSHOT
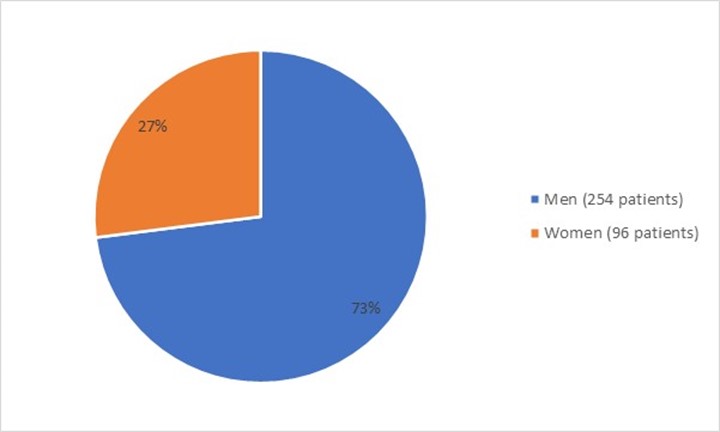
Figure 1 summarizes how many men and women were enrolled in the clinical trial used to evaluate the efficacy of FOTIVDA.
Figure 1: Baseline Demographics by Sex (Efficacy Population)
Source: FDA Review
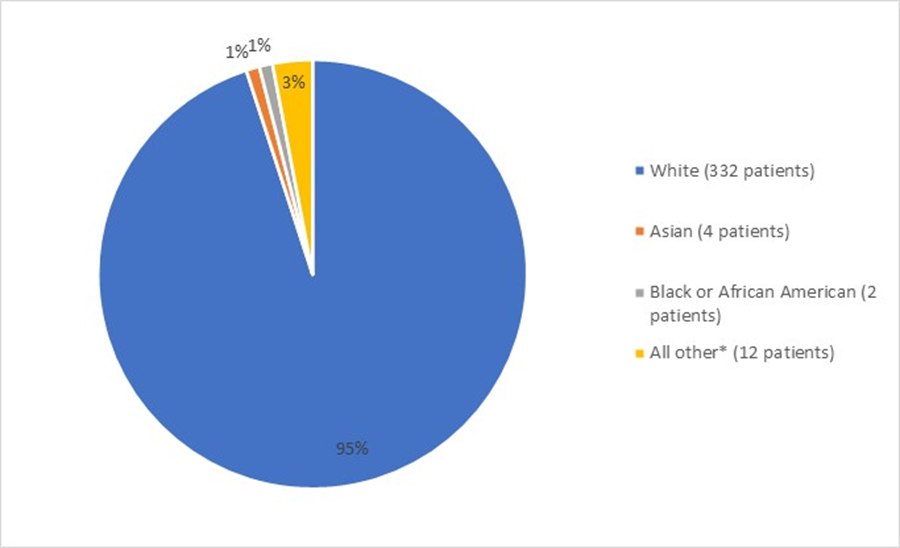
Figure 2 summarizes the percentage of patients by race enrolled in the clinical trial used to evaluate the efficacy of FOTIVDA.
Figure 2: Baseline Demographics by Race (Efficacy Population)
Source: FDA Review
*includes Decline to state, Unknown, and Other
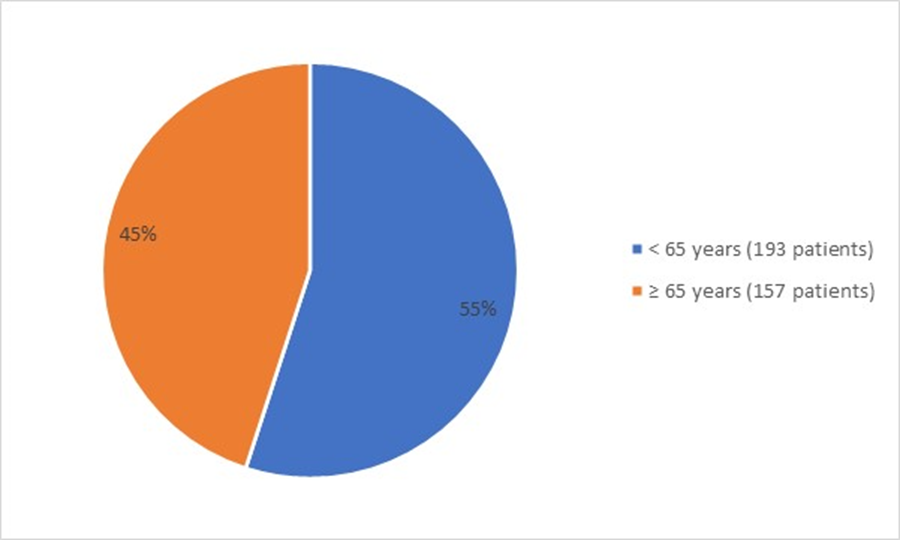
Figure 3 summarizes the percentage of patients by age group enrolled in the clinical trial used to evaluate the efficacy of FOTIVDA.
Figure 3: Baseline Demographics by Age (Efficacy Population)
Source: FDA Review
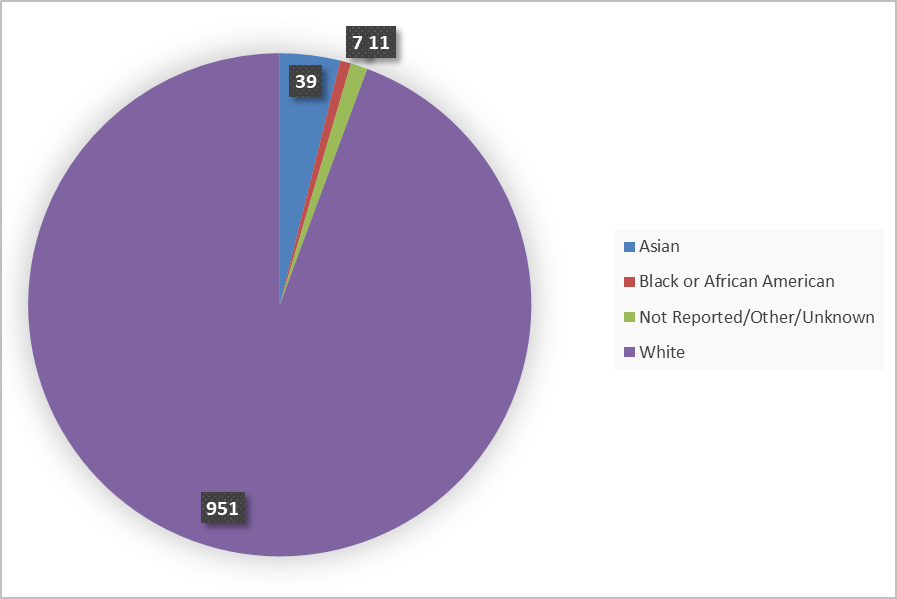
Figure 4 summarizes how many patients by race were in the combined trials used to evaluate the side effects of FOTIVDA.
Figure 4: Baseline Demographics by Race (Safety Population)
Source: FDA Review
Who participated in the trials?
More than twice as many men compared to women participated in this trial. This is expected because RCC occurs more frequently in men. The incidence of kidney cancer is relatively high in Black or African American men in the U.S. However, most of the trial participants were in Europe, and several of these countries have low numbers of Black patients.
Table 7: Demographic Characteristic (efficacy population)
|
Number (%) of Subjects |
FOTIVDA |
Sorafenib |
Total |
|---|---|---|---|
|
Sex, n (%) |
|||
|
Women |
49 (28) |
47 (27) |
96 (27) |
|
Men |
126 (72) |
128 (73) |
254 (73) |
|
Race, n (%) |
|||
|
Asian |
2 (1) |
2 (1) |
4 (1) |
|
Black or African American |
0 |
2 (1) |
2 (<1) |
|
White |
165 (94) |
167 (95) |
332 (95) |
|
Other |
1 (<1) |
0 |
1 (<1) |
|
Unknown |
7 (4) |
4 (2) |
11 (3) |
|
Age (years) |
|||
|
Mean (standard deviation) |
62.3 (9.7) |
63.4 (9.9) |
62.9 (9.8) |
|
Median |
62 |
64 |
63 |
|
< 65 |
98 (56) |
95 (54) |
193 (55) |
|
≥ 65 |
77 (44) |
80 (46) |
157 (45) |
|
Ethnicity |
|||
|
Hispanic or Latino |
10 (6) |
10 (6) |
20 (6) |
|
Not Hispanic or Latino |
156 (89) |
162 (93) |
318 (91) |
|
Unknown |
9 (5) |
3 (2) |
12 (3) |
|
Region |
|||
|
North America |
31 (18) |
27 (15) |
58 (17) |
|
Europe |
144 (82) |
148 (85) |
292 (83) |
Source: FDA Review
How were the trials designed?
FOTIVDA was evaluated in one clinical trial of 350 adult patients with advanced kidney cancer (renal cell carcinoma or RCC) that had been treated with 2 or more prior medicines and has come back or did not respond to treatment.
The trial compared patients who were randomly assigned to receive either FOTIVDA or sorafenib. Both the patients and the health care providers knew which treatment was being given. The treatment continued until the disease progression or the side effects became too toxic.
The trial compared the length of time patients were alive without progression between the two groups.
How were the trials designed?
Patients were allowed to participate in the trial if they had been treated with 2 or more prior medicines for RCC, and at least 1 of these must have been in the same class of drugs as FOTIVDA and sorafenib (but not FOTIVDA or sorafenib). They also were required to have at least some clear cell (a subtype of RCC) in their biopsy diagnosis. The trial did not allow patients to participate who had untreated brain tumors, HIV infection, were taking immunosuppressive medications, or any of the following within the prior 6 months: heart problems, highly elevated blood pressure, bleeding, non-healing wound, certain gastrointestinal problems, or clots. Patients had CT or MRI scans every 8 weeks during the trial to evaluate for progression.
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo that is compared to the actual drug being tested.
CT: Computed tomography.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
HAND-FOOT SKIN REACTION: A common reaction that patients have when taking certain type of medication for RCC. The symptoms usually begin a few weeks after beginning therapy, and may include dry, thickened, peeling, cracked, painful, and/or red skin.
MRI: Magnetic resonance imaging.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
PROGRESSION: An increase in total tumor measurements by at least 20% compared to when the patient started taking a drug; or the appearance of a new tumor (metastasis) related to the cancer for which the patient was being treated.
PROGRESSION-FREE SURVIVAL: The length of time during which 1) the patient’s cancer does not meet the definition of “progression” (see above) AND 2) the patient remains alive.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.