Drug Trials Snapshots: KIMMTRAK
HOW TO USE THIS SNAPSHOT
The information provided in Snapshots highlights who participated in the key clinical trials that supported the original FDA approval of this drug, and whether there were differences among sex, race, age, and ethnic groups. The “MORE INFO” bar shows more detailed, technical content for each section. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of the drugs.
LIMITATIONS OF THIS SNAPSHOT
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your healthcare provider about the benefits and risks of a drug.
Snapshots are limited to the information available at the time of the original approval of the drug and do not provide information on who participated in clinical trials that supported later approvals for additional uses of the drug (if applicable). Refer to the KIMMTRAK Prescribing Information for all of the approved conditions of use of this drug (e.g., indication(s), population(s), dosing regimen(s), safety information).
KIMMTRAK (tebentafusp)
KIM-track
Immunocore Commercial LLC
Approval date: January 25, 2022
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
Kimmtrak is a drug used to treat adult patients with a type of eye cancer called uveal melanoma. It is to be used in patients who have a specific type of gene marker measured from blood called HLA-A*02:01. It should be used only in patients whose cancer cannot be removed by surgery or has spread to other parts of the body (metastatic).
How is this drug used?
KIMMTRAK is injected into a vein (intravenous infusion) by a healthcare provider once a week. It takes about 15 to 20 minutes to receive a KIMMTRAK infusion, and patients are hospitalized for at least 16 hours during and after the first three infusions.
Who participated in the clinical trials?
The FDA approved KIMMTRAK based on evidence from one clinical trial of 378 patients with metastatic uveal melanoma disease. The trial was conducted at 58 sites across 14 countries including Australia, Belgium, Canada, France, Germany, Italy, Netherlands, Poland, Russian Federation, Spain, Switzerland, Ukraine, United Kingdom, USA. This same clinical trial was used to assess efficacy and safety.
What are the benefits of this drug?
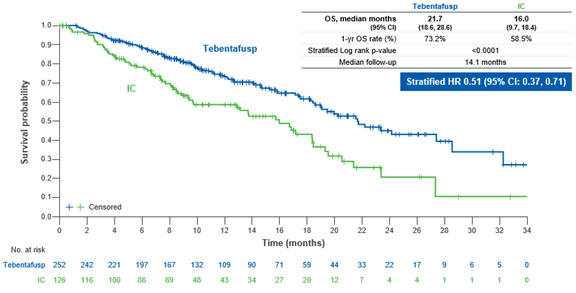
In the clinical study, patients treated with KIMMTRAK had a 49% greater likelihood to live longer versus patients that received other drugs. Patients receiving KIMMTRAK lived longer (average of 21.7 months) compared to patients taking other drugs (average of 16 months). Patients receiving KIMMTRAK also lived longer without their disease worsening (average of 3.3 months) compared to patients taking other drugs (average of 2.9 months).
What are the benefits of this drug?
The approval for KIMMTRAK was based upon a statistically significant improvement in overall survival (OS). Table 1 and Figure 1 summarize efficacy results from clinical trial in metastatic uveal melanoma patients.
Table 1: Efficacy Results of Clinical Trial
| KIMMTRAK (N=252) |
Investigator’s Choice (pembrolizumab, or ipilimumab, or dacarbazine) (N=126) |
|
|---|---|---|
| Overall Survival (OS) | ||
| Number of deaths | 87 (34.5%) | 63 (50%) |
| Median in months (95% CI) | 21.7 (18.6, 28.6) | 16 (9.7, 18.4) |
| HR (95% CI) | 0.51 (0.37, 0.71) | |
| p-value | <0.0001 | |
| Progression-free Survival | ||
| Number (%) of patients with event | 198 (78.6%) | 97 (77%) |
| Median in months (95% CI) | 3.3 (3, 5) | 2.9 (2.8, 3) |
| HR (95% CI) | 0.73 (0.58, 0.94) | |
| p-value | 0.0139 | |
CI: confidence interval
Adapted from KIMMTRAK Prescribing Information, Table 6
Figure 1 Kaplan-Meier Plot of Overall survival
Adapted from KIMMTRAK Prescribing information, Figure 1
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
- Sex: KIMMTRAK was similarly effective in men and women
- Race: The number of patients in races other than White was limited; therefore, differences in how well the drug worked among races could not be determined.
- Age: KIMMTRAK was similarly effective in patients younger and older than 65 years of age.
Were there any differences in how well the drug worked in clinical trials among sex, race, and age groups?
The table below summarizes OS results based on sex, and age.
Table 2: Overall Survival Results in Demographic Subgroups
| Demographic Subgroup | KIMMTRAK (N=252) |
Investigator’s Choice (N=126) | HR (95% CI) |
|---|---|---|---|
| Overall Deaths | 87/252 | 63/126 | 0.51 (0.37, 0.71) |
| Sex | |||
| Male | 48/128 | 35/62 | 0.48 (0.31, 0.75) |
| Female | 39/124 | 28/64 | 0.57 (0.35, 0.94) |
| Age Category | |||
| <65 years | 41/130 | 29/61 | 0.48 (0.30, 0.79) |
| >=65 years | 46/122 | 34/65 | 0.58 (0.38, 0.92) |
HR= hazard ratio; CI= confidence interval
What are the possible side effects?
KIMMTRAK may cause a serious and life-threatening side effect called cytokine release syndrome (large, rapid release of proteins into the blood from immune cells affected by KIMMTRAK) that usually happens shortly after the first three infusions. Other serious side effects include skin reactions and abnormal liver blood tests.
The most common side effects are cytokine release syndrome, rash, fever, itching, tiredness, nausea, chills, stomach pain, swelling, low blood pressure, dry skin, headache, vomiting, and abnormal liver blood tests.
What are the possible side effects (results of trials used to assess safety)?
The tables below summarize adverse reactions in patients with metastatic uveal melanoma in the trial.
Table 3: Adverse Reactions (≥20%) in Patients with Metastatic Uveal Melanoma Who Received KIMMTRAK
| Adverse Reactions | KIMMTRAK (N=245) |
Investigator’s Choice (pembrolizumab, or ipilimumab, or dacarbazine) (N=111) |
||
|---|---|---|---|---|
| All Grades (%) |
Grade 3 or 4 (%) |
All Grades (%) |
Grade 3 or 4 (%) |
|
| Immune system disorders | ||||
| Cytokine release syndromea | 89 | 0.8 | 2.7 | 0 |
| Skin and subcutaneous tissue disorders | ||||
| Rashb | 83 | 18 | 28 | 0 |
| Pruritus | 69 | 4.5 | 23 | 0 |
| Dry skin | 31 | 0 | 3.6 | 0 |
| Skin Hypopigmentationb | 28 | NA | 5 | NA |
| Erythema | 24 | 0 | 0.9 | 0 |
| Hair color changesb | 20 | NA | 0 | NA |
| General disorders and administration site conditions | ||||
| Pyrexia | 76 | 3.7 | 7 | 0.9 |
| Fatigueb | 64 | 6 | 42 | 0.9 |
| Chills | 48 | 0.4 | 3.6 | 0 |
| Edemab | 45 | 0 | 10 | 0 |
| Gastrointestinal disorders | ||||
| Nausea | 49 | 2 | 26 | 0.9 |
| Abdominal painb | 45 | 2.9 | 33 | 3.6 |
| Vomiting | 30 | 1.2 | 9 | 0 |
| Diarrhea | 25 | 1.2 | 20 | 2.7 |
| Vascular disorders | ||||
| Hypotension | 39 | 3.3 | 2.7 | 0 |
| Nervous system disorders | ||||
| Headache | 31 | 0.4 | 10 | 0.9 |
| Musculoskeletal and connective tissue disorders | ||||
| Arthralgia | 22 | 0.8 | 16 | 0 |
aRepresents algorithmic identification of CRS cases based on ASTCT grading criteria (Lee et al. 2019).
b Represents a composite of multiple related terms.
Adapted from KIMMTRAK Prescribing information, Table 4
Table 4: Selected Laboratory Abnormalities (≥ 10%) worsening from baseline in patients who received KIMMTRAK
| KIMMTRAKa (N = 245) |
Investigator’s Choicea (pembrolizumab, or ipilimumab, or dacarbazine) (N = 111) |
|||
|---|---|---|---|---|
| Grades 1-4 % |
Grades 3-4 % |
Grades 1-4 % |
Grades 3-4 % |
|
| HEMATOLOGY | ||||
| Lymphocyte count decreased | 91 | 56 | 26 | 1.8 |
| Hemoglobin decreased | 51 | 0.8 | 20 | 0.9 |
| Platelet count decreased | 16 | 0 | 15 | 0.9 |
| Neutrophil count decreased | 14 | 2 | 8 | 1.8 |
| CHEMISTRY | ||||
| Creatinine increased | 87 | 0.4 | 73 | 0 |
| Glucose increased | 66 | 3.3 | 39 | 4.6 |
| AST increased | 55 | 13 | 39 | 1.9 |
| ALT increased | 52 | 9 | 29 | 1.8 |
| Phosphate decreased | 51 | 11 | 20 | 2 |
| Albumin decreased | 47 | 2.1 | 14 | 0.9 |
| Calcium decreased | 45 | 1.6 | 15 | 1.9 |
| Lipase increased | 37 | 15 | 28 | 6 |
| Magnesium decreased | 34 | 0 | 8 | 0 |
| Alk phos increased | 34 | 2.9 | 36 | 1.8 |
| Sodium decreased | 30 | 2.9 | 15 | 0.9 |
| Potassium increased | 29 | 1.6 | 15 | 0.9 |
| Bilirubin increased | 27 | 4.1 | 14 | 7 |
| Amylase increased | 23 | 4.1 | 18 | 1 |
| Glucose decreased | 18 | 0.4 | 4.6 | 0 |
| Potassium decreased | 17 | 0.8 | 8 | 0.9 |
| Calcium increased | 13 | 0 | 3.7 | 0 |
Alk Phos = Alkaline Phosphatase; AST=aspartate aminotransferase; ALT=alanine aminotransferase
aThe denominator used to calculate the rate varied from 242 to 245 for KIMMTRAK and 105 to 109 for IC based on the number of patients with a baseline value and at least one post-treatment value for the laboratory assessment.
Adapted from KIMMTRAK prescribing information, Table 5
Were there any differences in side effects among sex, race and age?
- Sex: The occurrence of side effects was similar in men and women
- Race: The number of patients in races other than White was limited; therefore, differences in side effects among races could not be determined.
- Age: The occurrence of side effects was similar in patients younger and older than 65 years of age.
Were there any differences in side effects of the clinical trials among sex, race, and age groups?
The table below summarizes adverse events of special interest (AESI) by sex, age groups, and race
Table 5: Incidence Rates for Adverse Events of Special Interest in Demographic Subgroups
| Demographic Subgroup | Adverse Event of Special Interest | ||
|---|---|---|---|
| Cytokine Release Syndrome | Skin Toxicities | LFT Elevations | |
| Overall | 344/391 (88%) | 379/410 (92%) | 152/410 (37%) |
| Sex | |||
| Male | 169/197 (86%) | 190/209 (91%) | 77/209 (37%) |
| Female | 175/194 (90%) | 189/201 (94%) | 75/201 (37%) |
| Age Category | |||
| <65 years | 200/222 (90%) | 221/235 (94%) | 90/235 (38%) |
| >=65 years | 144/169 (85%) | 158/175 (90%) | 62/175 (35%) |
| Race | |||
| White | 318/361 (88%) | 348/379 (92%) | 133/379 (35%) |
| All other | 26/30 (87%) | 31/31 (100%) | 19/31 (61%) |
WHO WAS IN THE CLINICAL TRIALS?
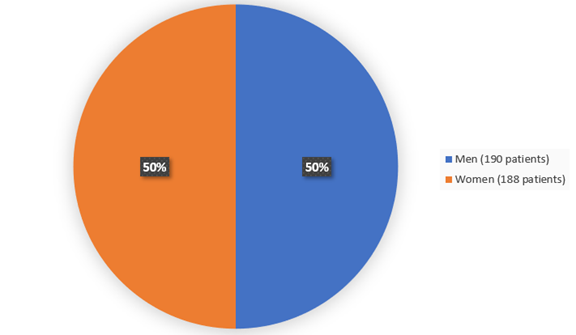
Figure 2 summarizes how many males and females were enrolled in the clinical trial. Evaluated for safety and efficacy.
Figure 2. Baseline Demographics by Sex
Adapted from FDA review
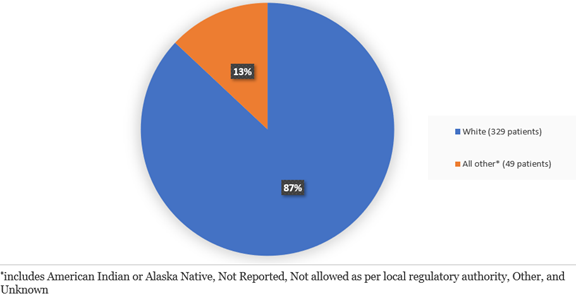
Figure 3 summarizes the percentage of patients by race enrolled in the clinical trial.
Figure 3. Baseline Demographics by Race
Adapted from FDA review
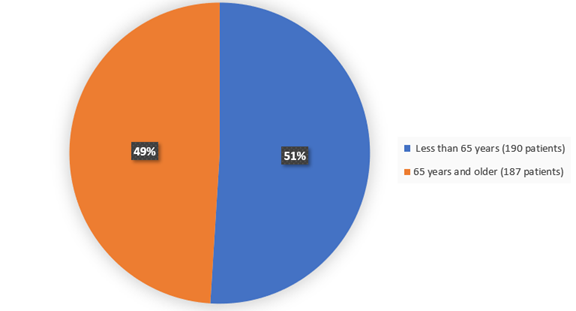
The figure below summarizes how many patients by age groups were in the clinical trial.
Figure 4. Baseline Demographics by Age
Adapted from FDA review
The table below summarizes baseline demographics for patients in the trial.
Table 6: Baseline Demographics for the Clinical Trial
| Characteristic | Study 202 (1L) | |
|---|---|---|
| Tebentafusp (N = 252) |
Investigator’s Choice (N = 126) |
|
| Age, years | ||
| n | 252 | 126 |
| Mean (Std) | 61.3 (11.9) | 63.6 (10.7) |
| Median (Min, Max) | 63.5 (23, 92) | 65.5 (25, 88) |
| Gender, n (%) | ||
| Female | 124 (49.2) | 64 (50.8) |
| Male | 128 (50.8) | 62 (49.2) |
| Race, n (%) | ||
| White | 222 (88.1) | 107 (84.9) |
| All others | 30 (9.1) | 18 (11.1) |
| Ethnicity (n, %) | ||
| Hispanic or Latino | 3 (1.2) | 6 (4.8) |
| Not Hispanic or Latino | 217 (86.1) | 102 (81.0) |
| Not reported | 29 (11.5) | 16 (12.7) |
| Unknown | 3 (1.2) | 2 (1.6) |
| Other | 0 | 0 |
Adapted from FDA review
How were the trials designed?
The benefits and side effects of KIMMTRAK were evaluated in one clinical trial of 378 patients with metastatic uveal melanoma. Two thirds of the patients were treated with KIMMTRAK and one third of the patients were treated with comparator drugs based on investigators’ choice that included pembrolizumab, ipilimumab or dacarbazine. The benefit of KIMMTRAK was evaluated by measuring how long patients lived after starting treatment with KIMMTRAK compared with patients who received comparator drugs.
How were the trials designed?
The efficacy and safety of KIMMTRAK were primarily evaluated in one open-label, randomized, multicenter clinical trial. The trial enrolled adult patients who were 18 years and older with previously untreated metastatic uveal melanoma. Patients with clinically significant cardiac disease or the presence of symptomatic or untreated brain metastasis were excluded. Treatment continued until disease progression, or the healthcare provider determined there was no longer benefit, or for unacceptable toxicity. Randomization was stratified by lactate dehydrogenase (LDH) level at study entry. Patients were randomized (2:1) to receive KIMMTRAK weekly by intravenous infusion administered at 20 mcg intravenously on Day 1, 30 mcg intravenously on Day 8, 68 mcg intravenously on Day 15, and 68 mcg intravenously once every week thereafter (N=252) or Investigator’s choice (N=126) of pembrolizumab, ipilimumab, or dacarbazine.
The major efficacy outcome was overall survival (OS). An additional efficacy outcome was investigator-assessed progression free survival (PFS) per RECIST 1.1.
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY:How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO:An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP:A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.
PRESCRIBING INFORMATION