Drug Trials Snapshots: RYLAZE
HOW TO USE THIS SNAPSHOT
The information provided in Snapshots highlights who participated in the key clinical trials that supported the original FDA approval of this drug, and whether there were differences among sex, race, age, and ethnic groups. The “MORE INFO” bar shows more detailed, technical content for each section. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of the drugs.
LIMITATIONS OF THIS SNAPSHOT
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your healthcare provider about the benefits and risks of a drug.
Snapshots are limited to the information available at the time of the original approval of the drug and do not provide information on who participated in clinical trials that supported later approvals for additional uses of the drug (if applicable). Refer to the RYLAZE Prescribing Information for all of the approved conditions of use of this drug (e.g., indication(s), population(s), dosing regimen(s), safety information).
RYLAZE (asparaginase erwinia chrysanthemi (recombinant)-rywn)
(Rye layz’)
Jazz Pharmaceuticals Ireland Limited
Original Approval date: June 30, 2021
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
RYLAZE is used with other chemotherapy drugs for the treatment of acute lymphoblastic leukemia (ALL) and lymphoblastic lymphoma (LBL) in adult and children 1 month or older who have developed an allergic reaction (hypersensitivity) to E. coli-derived asparaginase.
ALL is a rapidly progressing cancer that forms in the bone marrow and results in an increased number of leukemia cells (lymphoblasts, a type of white blood cell) in the bloodstream.
LBL is a rare, rapidly progressing cancer of immature lymphocytes (lymphoblasts), most often seen in teenagers and young adults.
How is this drug used?
RYLAZE is given as an injection into the muscle. The number of injections and duration of treatment are determined by the healthcare provider.
Who participated in the clinical trials?
The FDA approval of RYLAZE was based on evidence from one ongoing clinical trial (NCT04145531) in 102 children and adult patients with a type of cancer called ALL/LBL. These patients had developed allergy to another type of asparaginase.
The trial was conducted in 67 sites across the United States and Canada.
Data for the evaluation of benefit (efficacy) is from 101 patients treated with RYLAZE in this trial. Demographics of the efficacy population is presented below in Figure 1, Figure 3, Figure 5, and Figure 7.
Data for the evaluation of side effects of RYLAZE (safety) is from a subset of 33 patients. Demographics of the safety population that provided data for side effects of RYLAZE is presented below in Figure 2, Figure 4, Figure 6, and Figure 8.
The number of patients representing efficacy findings may differ from the number of patients representing safety findings due to different pools of study participants analyzed for efficacy and safety.
What are the benefits of this drug?
RYLAZE is a type of medication called asparaginase. Asparaginase is an important medication used in patients with ALL and LBL that breaks down the amino acid asparagine. Leukemic cells are unable to make their own asparagine and die when asparagine is removed by RYLAZE.
RYLAZE is a different type of asparaginase that can be used in patients that develop allergy to another asparaginase. This is how this medication helps leukemia patients.
What are the benefits of this drug (results of trials used to assess efficacy)?
The measurement of Nadir Serum Asparaginase Activity (NSAA levels) ≥ 0.1 U/mL is the accepted threshold to demonstrate adequate asparagine depletion and clinical efficacy of asparaginase. Asparagine removal from the blood can be measured by serum asparaginase activity to indicate effectiveness of RYLAZE.
The determination of effectiveness in this trial was based on a demonstration of the achievement and maintenance of NSAA above the level of 0.1 U/mL. The results of modeling and simulations showed that for a dosage of 25 mg/m2 administered intramuscularly every 48 hours, the proportion of patients maintaining NSAA ≥ 0.1 U/mL at 48 hours after a dose of RYLAZE was 93.6% (95% CI: 92.6%, 94.6%).
Source: RYLAZE Prescribing Information
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
- Sex: RYLAZE worked similarly in males and females.
- Race: RYLAZE worked similarly across different races and ethnicities.
- Age: RYLAZE worked similarly in children and adult patients.
Were there any differences in how well the drug worked in clinical trials among sex, race, and age groups?
Evidence of effectiveness was obtained from the ongoing pivotal trial (NCT04145531), based on a combination of observed data and Population Pharmacokinetic (PPK) modeling and simulation. Subgroup efficacy analysis was not assessed in this trial. FDA used the Population PK modeling to assess the influence of sex, race, and age. The conclusions made above are based on the FDA PPK analysis as summarized below:
- Sex was not a significant covariate in the population PK model, suggesting that there are no significant differences in RYLAZE exposure between men and women.
- The influence of race and ethnicity on the PK of RYLAZE was evaluated within the population PK analysis. Regardless of race or ethnicity, at least 90% of patients are predicted to meet the primary endpoint (NSAA >0.1 IU/mL) after administration of the recommended dosage regimen, suggesting that there are no significant differences in RYLAZE exposure based on race or ethnicity.
- The influence of age on the PK of RYLAZE was evaluated within the population PK analysis. Results are described in Table 1 below. Overall, Rylaze worked similarly in patients from 1 month to 80 years. For all age groups, SAA profiles were similar and proportion of patients with predicted NSAA ≥0.1 IU/mL was >90%.
Table 1: FDA's Simulated Median and 95% Confidence Intervals of Response Rate of Achieving Therapeutic NSAA Levels in Subjects Following 25 mg/m2 Every 48 Hours for 7 Doses per 2-Week Course Stratified by Six Age Groups
| Dosing Regimen | Age group | Simulated response rate Median [95% Cl] |
|---|---|---|
| 25 mg/m2 every 48 hours for 7 doses per 2-week course | 1 month - <2 years of age | 96.8% [94.2%, 98.7%) |
| 2 - <6 years of age | 96.0% [93.1%, 98.0%] | |
| 6 - <12 years of age | 94.5% [91.8%, 96.6%] | |
| 12 - <18 years of age | 93.3% [89.6%, 95.5%] | |
| 18 - <65 years of age | 92.6% [90.8%, 94.5%] | |
| 65 - <80 years of age | 92.5% [89.7%, 95.4%) |
Source: Adapted from FDA Review
What are the possible side effects?
RYLAZE may cause serious allergic reactions, inflammation of pancreas, blood clots, bleeding, or liver damage.
The most common side effects of RYLAZE when given in combination with chemotherapy for the treatment of ALL and LBL are abnormal liver tests, nausea, muscle and bone pain, and fatigue.
What are the possible side effects (results of trials used to assess safety)?
The table below summarizes adverse reactions in the clinical trial.
Table 2: Adverse Reactions (≥ 15% incidence) in Patients Receiving RYLAZE 25 mg/m2 as a Component of Multi-Agent Chemotherapy in Study JZP458-201
| Adverse Reaction | RYLAZE 25 mg/m2 Dosagea N=33 |
|
|---|---|---|
| All Grades (%) |
Grades 3-4 (%) |
|
| Abnormal liver test* | 70 | 12 |
| Nausea* | 46 | 9 |
| Musculoskeletal pain* | 39 | 6 |
| Fatigue* | 36 | 3 |
| Infection*b | 30 | 12 |
| Headache | 30 | 0 |
| Pyrexia | 27 | 6 |
| Drug hypersensitivity* | 24 | 6 |
| Febrile neutropenia | 24 | 24 |
| Decreased appetite | 21 | 6 |
| Stomatitis | 21 | 9 |
| Bleeding* | 21 | 0 |
| Hyperglycemia | 21 | 3 |
| Abdominal pain* | 18 | 0 |
| Tachycardia* | 18 | 0 |
| Diarrhea* | 18 | 6 |
| Constipation | 15 | 0 |
| Dehydration | 15 | 9 |
| Neuropathy peripheral* | 15 | 0 |
| Cough | 15 | 0 |
| Insomnia | 15 | 0 |
*Includes grouped terms
Grading is based on Common Terminology Criteria for Adverse Events version 5.0
a RYLAZE was administered as a component of multi-agent chemotherapy regimens.
b Does not include the following fatal adverse reactions: infection (N=1).
Safety data for patients treated on a Monday, Wednesday, and Friday schedule.
RYLAZE Prescribing Information
Were there any differences in side effects among sex, race and age?
- Sex: The differences in side effects among sex could not be determined.
- Race: The number of patients of races other than White was small; therefore, differences in side effects among races could not be determined.
- Age: The number of adult patients was small; therefore differences in side effects among races could not be determined.
Demographics Snapshot
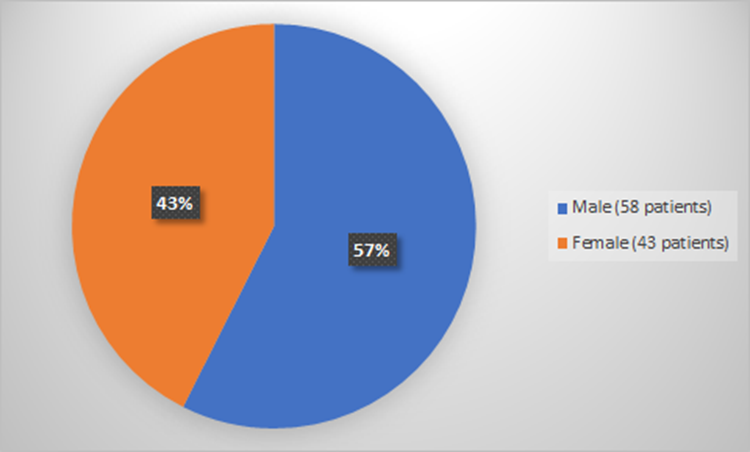
Figure 1 summarizes how many men and women were enrolled in the clinical trial used to evaluate the efficacy of RYLAZE.
Figure 1. Baseline Demographics by Sex (Evaluation of Efficacy)
Source: Adapted from FDA Review
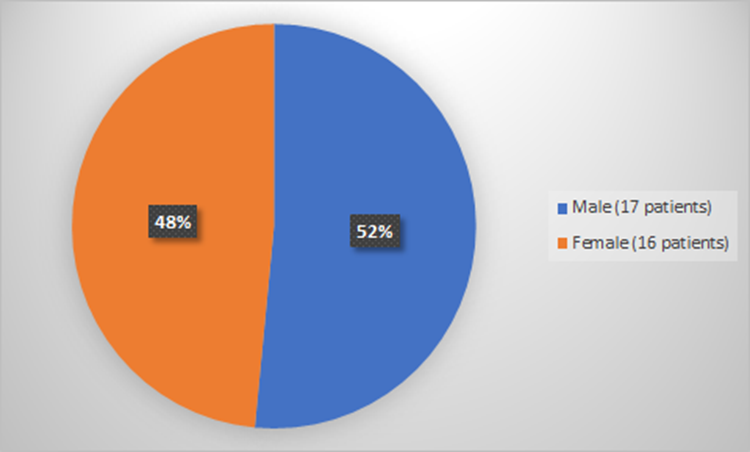
Figure 2 summarizes how many patients by sex were used to evaluate the side effects of RYLAZE.
Figure 2. Baseline Demographics by Sex (Evaluation of Safety)
Source: Adapted from FDA Review
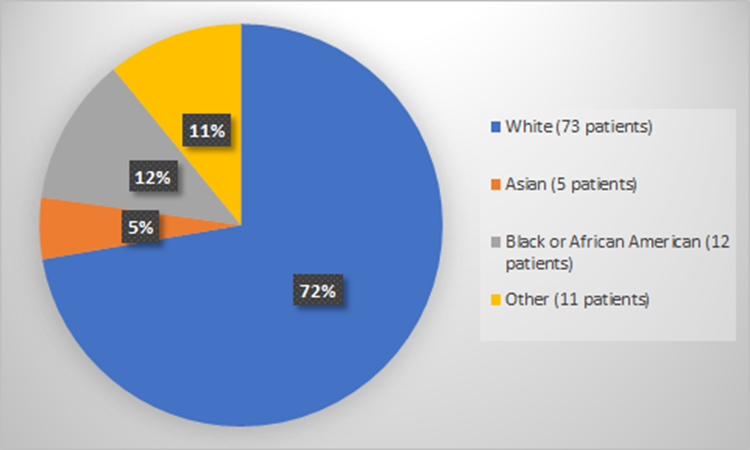
Figure 3 summarizes how many patients by race enrolled in the clinical trial used to evaluate the efficacy of RYLAZE.
Figure 3. Baseline Demographics by Race (Evaluation of Efficacy)
*Other includes American Indian or Alaska Native, Multiple, or Not Reported
Source: Adapted from FDA Review
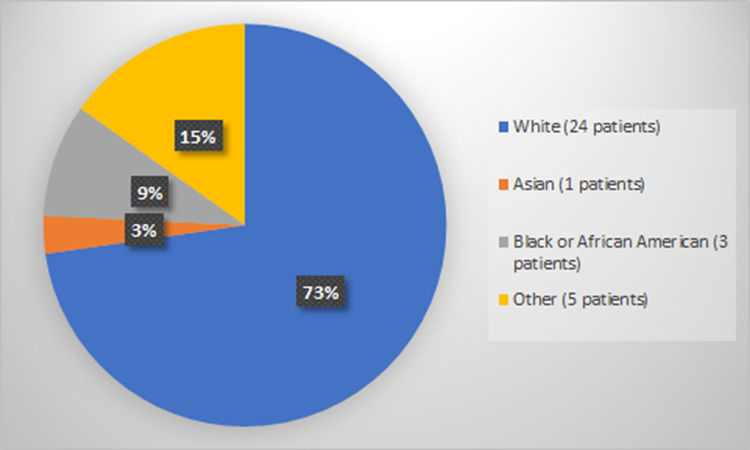
Figure 4 summarizes how many patients by race were used to evaluate the side effects of RYLAZE.
Figure 4. Baseline Demographics by Race (Evaluation of Safety)
*Other includes Multiple or Not Reported
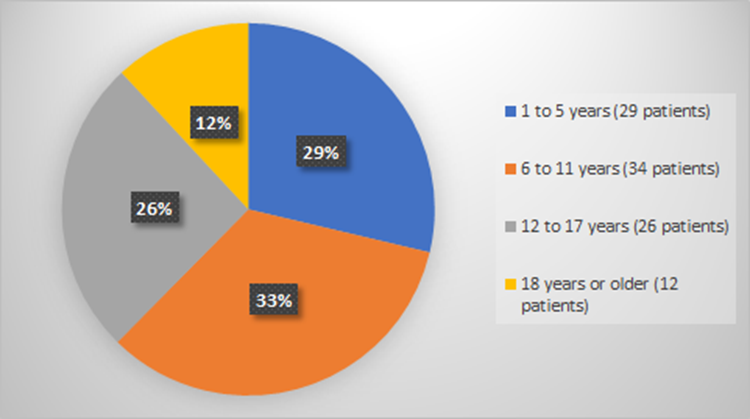
Figure 5 summarizes how many patients by age enrolled in the clinical trial used to evaluate the efficacy of RYLAZE.
Figure 5. Baseline Demographics by Age (Evaluation of Efficacy)
Source: Adapted from FDA Review
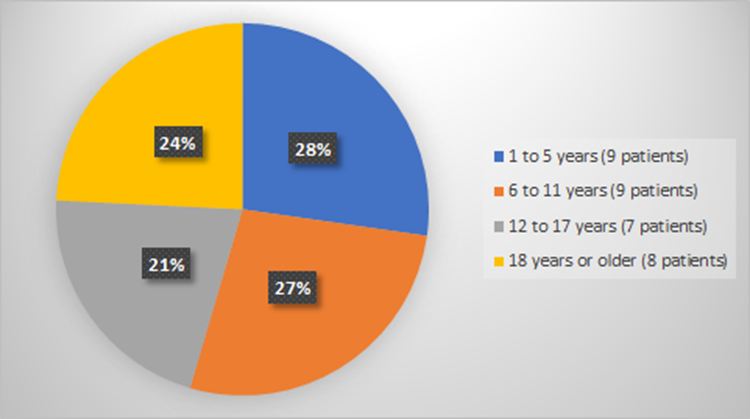
Figure 6 summarizes how many patients by age were used to evaluate the side effects of RYLAZE.
Figure 6. Baseline Demographics by Age (Evaluation of Safety)
Source: Adapted from FDA Review
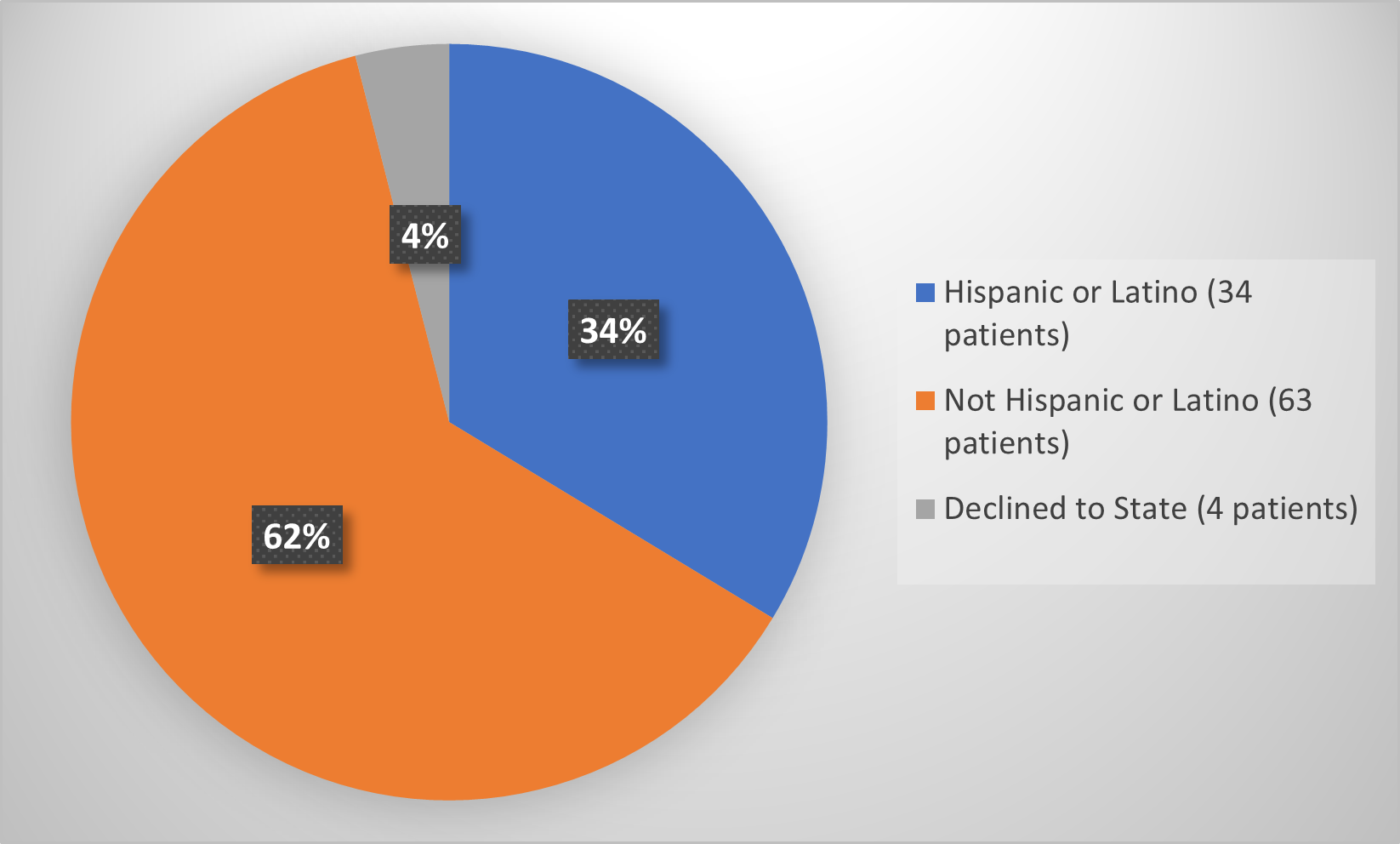
Figure 7 summarizes how many patients by ethnicity enrolled in the clinical trial used to evaluate the efficacy of RYLAZE.
Figure 7. Baseline Demographics by Ethnicity (Evaluation of Efficacy)
Source: Adapted from FDA Review
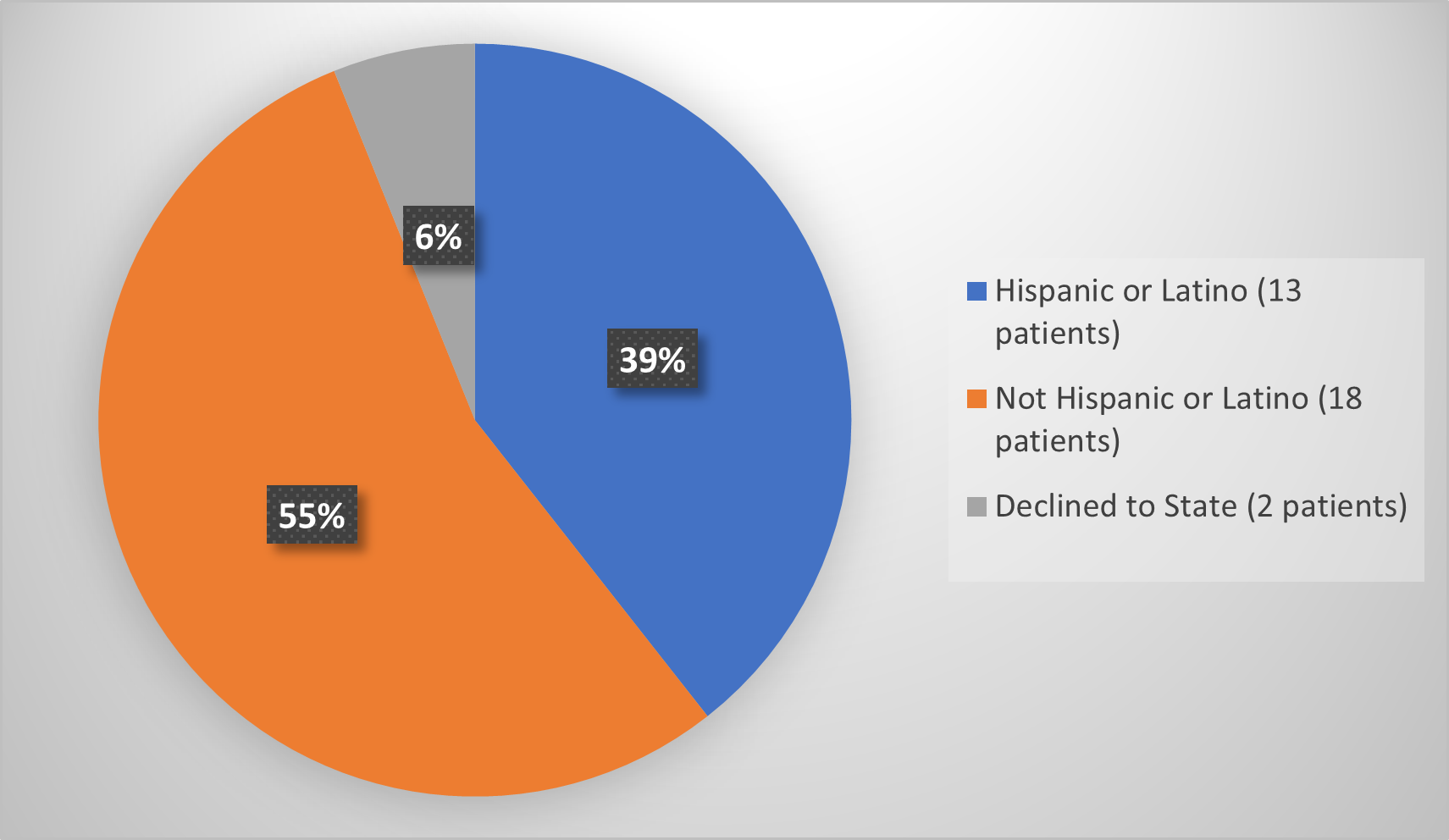
Figure 8 Summarizes how many patients by ethnicity were used to evaluate the side effects of RYLAZE.
Figure 8. Baseline Demographics by Ethnicity (Evaluation of Safety)
Source: Adapted from FDA Review
Who participated in the trials?
Table 3. Demographics by Race (Evaluation of Efficacy)
| Race | Number of Patients | Percentage |
|---|---|---|
| White | 73 | 72% |
| Black or African American | 12 | 12% |
| Asian | 5 | 5% |
| Not Reported | 9 | 9% |
| American Indian or Alaska Native | 1 | 1% |
| Multiple | 1 | 1% |
Source: Adapted from FDA Review
Table 4. Demographics by Race (Evaluation of Safety)
| Race | Number of Patients | Percentage |
|---|---|---|
| White | 24 | 73% |
| Black or African American | 3 | 9% |
| Asian | 1 | 3% |
| Not Reported | 4 | 12% |
| Multiple | 1 | 3% |
Source: Adapted from FDA Review
How were the trials designed?
The benefits and side effects of RYLAZE were evaluated from one ongoing clinical trial (NCT04145531).
Patients with ALL or LBL received RYLAZE after they developed allergy to an E.coli based long acting asparaginase.
The trial enrolled patients of all ages. All patients received RYLAZE as part of a combination of drugs used for treatment of ALL/LBL.
Patients received 6 doses of RYLAZE to make up for each dose of a prior long acting asparaginase therapy as prescribed by the doctor. Doses were given on a Monday, Wednesday, and Friday schedule for two consecutive weeks.
The benefit of RYLAZE was evaluated by measuring serum asparaginase levels at predefined time points after RYLAZE treatment.
How were the trials designed?
The safety and efficacy of RYLAZE were primarily evaluated in one pivotal ongoing open-label, multicenter dose confirmation and PK trial in children and adults.
Eligible patients had ALL or LBL and had developed allergy after treatment with an E. coli-derived asparaginase. A treatment course consisted of RYLAZE administered intramuscularly on a Monday, Wednesday, and Friday schedule for a total of 6 doses over 2 consecutive weeks.
The patients were treated in three cohorts (cohort 1a, 1b and 1c). Patients in cohort 1a received a dose of 25 mg/m2 on a Monday, Wednesday, and Friday dosing schedule, cohort 1b patients received a dose of 37.5 mg/m2 on a Monday, Wednesday, and Friday dosing schedule, and cohort 1c patients received a dose of 25 mg/m2 on Monday and Wednesday and 50 mg/m2 on Friday dosing schedule.
The key efficacy endpoint was the achievement and maintenance of last 72 hour and last 48hour NSAA level ≥ of 0.1 U/mL during the first course.
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.