Drug Trials Snapshots: TRUSELTIQ
HOW TO USE THIS SNAPSHOT
The information provided in Snapshots highlights who participated in the key clinical trials that supported the original FDA approval of this drug, and whether there were differences among sex, race, age, and ethnic groups. The “MORE INFO” bar shows more detailed, technical content for each section. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of the drugs.
LIMITATIONS OF THIS SNAPSHOT
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your healthcare provider about the benefits and risks of a drug.
Some of the information in this Snapshot is for presentation purposes and does not represent the approved conditions of use of this drug. Refer to the TRUSELTIQ Prescribing Information for all of the approved conditions of use of this drug (e.g., indication(s), population(s), dosing regimen(s), safety information).
Snapshots are limited to the information available at the time of the original approval of the drug and do not provide information on who participated in clinical trials that supported later approvals for additional uses of the drug (if applicable).
TRUSELTIQ (infigratinib)
Tru ‘sel tik
QED Therapeutics Inc.
Approval date: May 28, 2021
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
TRUSELTIQ is a drug used for the treatment of adults with previously treated bile duct cancer (cholangiocarcinoma) that is advanced, unresectable (cannot be removed surgically) or has spread to other parts of the body (metastatic). It should be used in patients who have been previously treated with chemotherapy and whose cancer has a certain type of abnormality in the fibroblast growth factor receptor (FGFR) gene.
How is this drug used?
TRUSELTIQ is a capsule that is taken by mouth once daily for 21 days followed by 7 days off treatment to complete a 28-day treatment cycle.
Who participated in the clinical trials?
The FDA approved TRUSELTIQ based on evidence from one clinical trial (NCT02150967) of 108 patients with bile duct cancer (cholangiocarcinoma). The trials were conducted at 18 sites in the United States, Europe, and Asia.
What are the benefits of this drug?
In the trial, 23% (25 of 108) of patients treated with TRUSELTIQ achieved partial or complete shrinkage of the tumor (overall response rate). Of these patients, 32% had tumor shrinkage lasting 6 months or longer and 4% had tumor shrinkage lasting 12 months or longer.
TRUSELTIQ was approved under the FDA’s accelerated approval program, which provides earlier patient access to a promising new drug while the company continues to conduct clinical trials to confirm that the drug works well.
What are the benefits of this drug (results of trials used to assess efficacy)?
Table 1 shows efficacy results based on the overall response rate (ORR) and duration of response (DOR) as determined by a blinded independent review committee (BIRC) according to guidelines for determining tumor shrinkage known as Response Evaluation Criteria in Solid Tumors (RECIST) v 1.1.
Table 1. Efficacy Results
| Efficacy Parameter | TRUSELTIQ N=108 |
|---|---|
| ORR (95% CI)a | 23% (16%, 32%) |
| CR | 1 (1%) |
| PR | 24 (22%) |
| Median DOR (95% CI)a | 5.0 (3.7%, 9.3%) |
| DOR ≥6 months | 8 (32%) |
| DOR ≥12 months | 1 (4%) |
Source: Adapted from FDA review
a The 95% confidence interval (CI) for ORR was calculated using the Clopper-Pearson exact method; 95% CI for Median DOR was calculated using the Brookmeyer-Crowley method.
Note: Data are from BIRC per RECIST v1.1, and complete and partial responses are confirmed.
Abbreviations: BIRC, blinded independent review committee; CR, complete response; DOR, duration of response; ORR, overall response rate; PR, partial response; RECIST, Response Evaluation Criteria in Solid Tumors
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
- Sex: TRUSELTIQ appears to work similarly in males and females.
- Race: The number of patients of races other than White was small; therefore, differences in how TRUSELTIQ worked among races could not be determined.
- Age: TRUSELTIQ appears to work similarly in patients below and above 65 years of age.
Were there any differences in how well the drug worked in clinical trials among sex, race, and age groups?
Table 2 summarizes efficacy results by relevant demographic subgroups.
Table 2. ORR Per RECIST 1.1 as Assessed by BIRC for Subgroups
| Characteristics (n) N=108 |
Response Rate (95% CI) |
|---|---|
| Sex | |
| Male (41) | 27 (14, 43) |
| Female (67) | 21 (12, 33) |
| Race | |
| White (78) | 24 (15, 35) |
| All other (30) | 20 (8, 39) |
| Age group | |
| <65 years (82) | 22 (14, 32) |
| ≥65 years (26) | 27 (12, 48) |
| Region | |
| North America (77) | 29 (19, 40) |
| Other (31) | 10 (2, 26) |
Source: Adapted from FDA review
Abbreviations: BIRC, blinded independent review committee; CI, confidence interval; ORR, overall response rate; RECIST, Response Evaluation Criteria in Solid Tumors
What are the possible side effects?
TRUSELTIQ may cause serious side effects including detachment of retina (inner layer of the eye), increased phosphate level in the blood, and harm to an unborn baby.
The most common side effects of TRUSELTIQ are increased phosphate level in the blood, increased creatinine levels in the blood, nail changes, mouth sores, dry eye, fatigue, alopecia, and palmar-plantar erythrodysesthesia (rash, redness, pain, swelling or blisters on the palms of the hands or soles of the feet ).
What are the possible side effects (results of trials used to assess safety)?
Table 3 summarizes adverse reactions that occurred in ≥15% of patients treated with TRUSELTIQ.
Table 3. Adverse Reactions in ≥15% of Patients Who Received TRUSELTIQ
| Adverse Reaction | TRUSELTIQ N=108 |
|
|---|---|---|
| All Grades (%) |
Grade 3 or 4 a (%) |
|
| Skin and subcutaneous tissue disorders | ||
| Nail toxicityb | 57 | 2* |
| Alopecia | 38 | 0 |
| Palmar-plantar erythrodysesthesia syndrome | 33 | 7* |
| Dry skin | 23 | 0 |
| Gastrointestinal disorders | ||
| Stomatitisc | 56 | 15* |
| Constipation | 30 | 1* |
| Abdominal paind | 26 | 5* |
| Dry mouth | 25 | 0 |
| Diarrhea | 24 | 3* |
| Vomiting | 21 | 1* |
| Nausea | 19 | 1* |
| Dyspepsia | 17 | 0 |
| Eye disorderse | ||
| Dry eyef | 44 | 0 |
| Eyelash changesg | 25 | 0 |
| Vision blurred | 21 | 0 |
| General disorders and administrative site conditions | ||
| Fatigueh | 44 | 4* |
| Edemai | 17 | 1* |
| Pyrexia | 15 | 1* |
| Musculoskeletal and connective tissue disorders | ||
| Arthralgia | 32 | 0 |
| Pain in extremity | 17 | 2* |
| Nervous system disorders | ||
| Dysgeusia | 32 | 0 |
| Headache | 17 | 1* |
| Metabolism and nutrition disorders | ||
| Decreased appetite | 22 | 1* |
| Respiratory, thoracic and mediastinal disorders | ||
| Epistaxis | 18 | 0 |
| Investigations | ||
| Weight decreased | 15 | 2* |
Source: Adapted from FDA Review
Graded according to National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE 4.03).
a Events of Grade 3 only (no Grade 4 occurred) are marked with an asterisk.
b Includes ingrown nail, nail bed bleeding, nail bed disorder, nail bed inflammation, nail bed tenderness, nail discoloration, nail disorder, nail dystrophy, nail hypertrophy, nail infection, nail ridging, onychalgia, onychoclasis, onycholysis, onychomadesis, onychomycosis, and paronychia.
c Includes mouth ulceration and stomatitis.
d Includes abdominal pain, abdominal pain upper, abdominal discomfort, and abdominal pain lower.
e Severity of eye disorders is not represented by CTCAE Grading.
f Includes dry eye, keratitis, lacrimation increased, pinguecula, and punctate keratitis.
g Includes blepharitis, eyelash changes, eyelash discoloration, growth of eyelashes, trichiasis, and trichomegaly.
h Includes asthenia and fatigue.
I Includes edema peripheral and edema.
Table 4 summarizes select laboratory abnormalities worsening from baseline in patients receiving TRUSELTIQ that occurred in ≥15% of patients treated with TRUSELTIQ.
Table 4. Select Laboratory Abnormalities (≥15%) Worsening From Baseline in Patients Receiving TRUSELTIQ
| Laboratory Abnormality | TRUSELTIQ N=108 |
|
|---|---|---|
| All Grades (%) |
Grade 3 or 4 (%) |
|
| Hematology | ||
| Decreased hemoglobin | 53 | 5 |
| Decreased lymphocytes | 43 | 9 |
| Decreased platelets | 37 | 4 |
| Decreased leukocytes | 26 | 3 |
| Chemistry | ||
| Increased creatinine | 93 | 7 |
| Increased phosphate a | 90 | 13 |
| Decreased phosphate | 64 | 31 |
| Increased alkaline phosphatase | 54 | 8 |
| Increased alanine aminotransferase | 51 | 6 |
| Increased lipase | 44 | 7 |
| Increased calcium | 43 | 7 |
| Decreased sodium | 41 | 20 |
| Increased triglycerides | 38 | 3 |
| Increased aspartate aminotransferase | 38 | 4 |
| Increased urate | 37 | 37 |
| Decreased albumin | 24 | 1 |
| Increased bilirubin | 24 | 6 |
| Decreased potassium | 21 | 3 |
| Increased cholesterol | 18 | 1 |
| Increased potassium | 17 | 3 |
Source: Adapted from FDA Review
The denominator used to calculate the rate varied from 104 to 107 based on the number of patients with a baseline value and at least one post-treatment value. These laboratory abnormalities are values that reflect worsening from baseline. Graded per NCI CTCAE 4.03.
a NCI CTCAE 4.03 does not define grades for increased phosphate. Laboratory value shift table categories were used to assess increased phosphorus levels (Grades ≥3 defined as ≥9mg/dL).
Abbreviations: NCI CTCAE, National Cancer Institute Common Terminology Criteria for Adverse Events
Were there any differences in side effects among sex, race and age?
- Sex: The occurrence of side effects appeared similar in males and females.
- Race: The number of patients of races other than White was small; therefore, differences in how TRUSELTIQ worked among races could not be determined.
- Age: The occurrence of side effects appeared similar in patients below and above 65 years of age.
Were there any differences in side effects of the clinical trials among sex, race, and age groups?
Analyses of side effects by subgroups are presented below.
Table 5. Summary of Adverse Events by Sex
| Adverse Reaction | TRUSELTIQ N=108 |
|
|---|---|---|
| Male N=41 n (%) |
Female N=67 n (%) |
|
| All Grade AEs | 41 (100) | 66 (99) |
| Grade 3 or 4 AEs | 27 (66) | 44 (66) |
Source: Adapted from FDA Review
Abbreviations: AE, adverse event
Table 6. Summary of Adverse Events by Race
| Adverse Reaction | TRUSELTIQ N=108 |
|||
|---|---|---|---|---|
| White N=78 n (%) |
Black N=4 n (%) |
Asian N=11 n (%) |
All Other N=15 n (%) |
|
| All Grade AEs | 77 (99) | 4 (100) | 11 (100) | 10 (67) |
| Grade 3 or 4 AEs | 53 (68) | 4 (100) | 3 (27) | 8 (53) |
Source: Adapted from FDA Review
Abbreviations: AE, adverse event
Table 7. Summary of Adverse Events (AEs) by Age
| Adverse Reaction | TRUSELTIQ N=108 |
|
|---|---|---|
| <65 Years N=82 n (%) |
≥65 Years N=26 n (%) |
|
| All Grade AEs | 81 (99) | 26 (100) |
| Grade 3 or 4 AEs | 53 (65) | 20 (77) |
Source: Adapted from FDA Review
Abbreviations: AE, adverse event
DEMOGRAPHICS SNAPSHOT
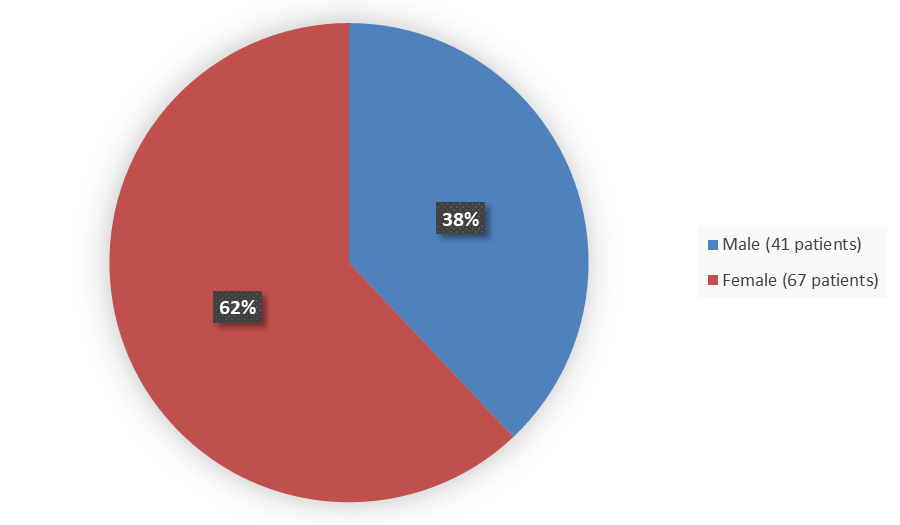
Figure 1 summarizes how many men and women were enrolled in the clinical trial used to evaluate the safety and efficacy of TRUSELTIQ.
Figure 1. Baseline Demographics by Sex (Safety and Efficacy Population)
Adapted from FDA review
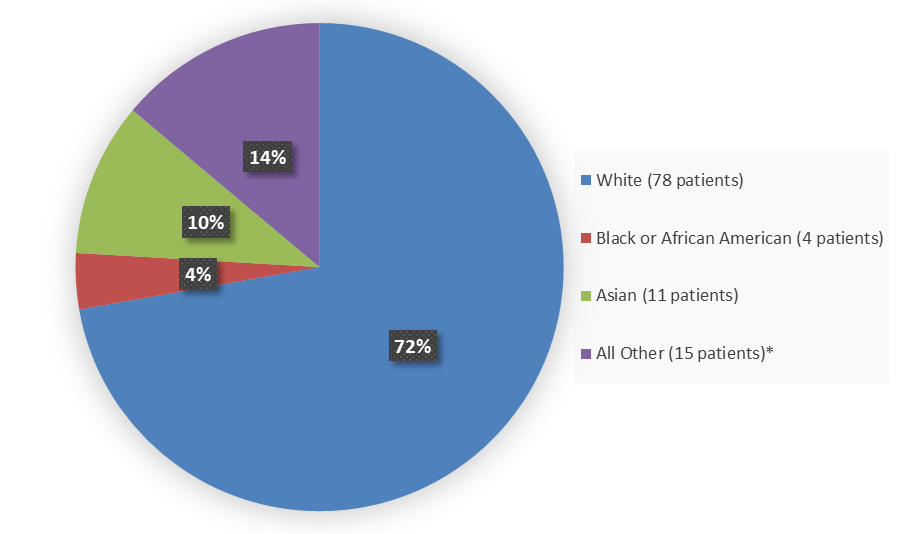
Figure 2 summarizes how many patients by Race were enrolled in the combined trials used to evaluate the side effects of TRUSELTIQ.
Figure 2. Baseline Demographics by Race (Safety and Efficacy Population)
Source: Adapted from FDA review.
* Includes Other and Missing
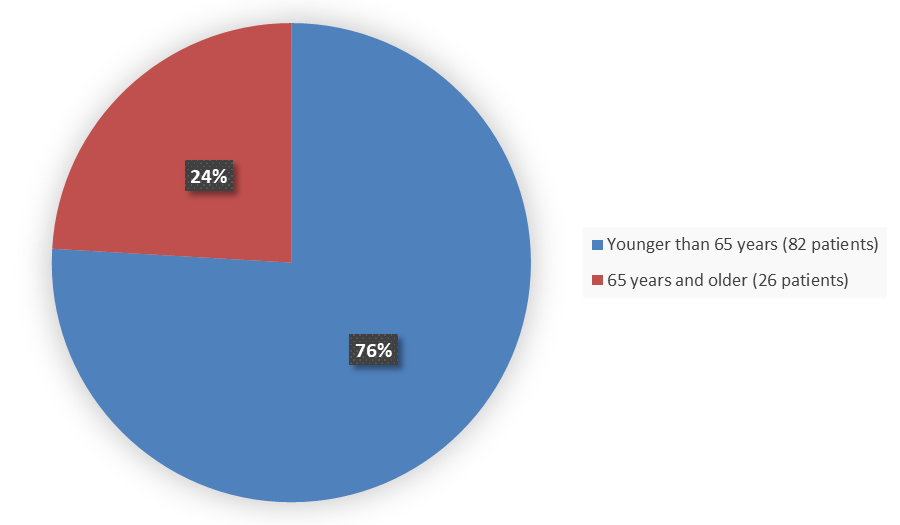
Figure 3 summarizes how many patients by age were enrolled in the trial used to evaluate the side effects of TRUSELTIQ.
Figure 3. Baseline Demographics by Age (Safety Population)
Source: Adapted from FDA Review
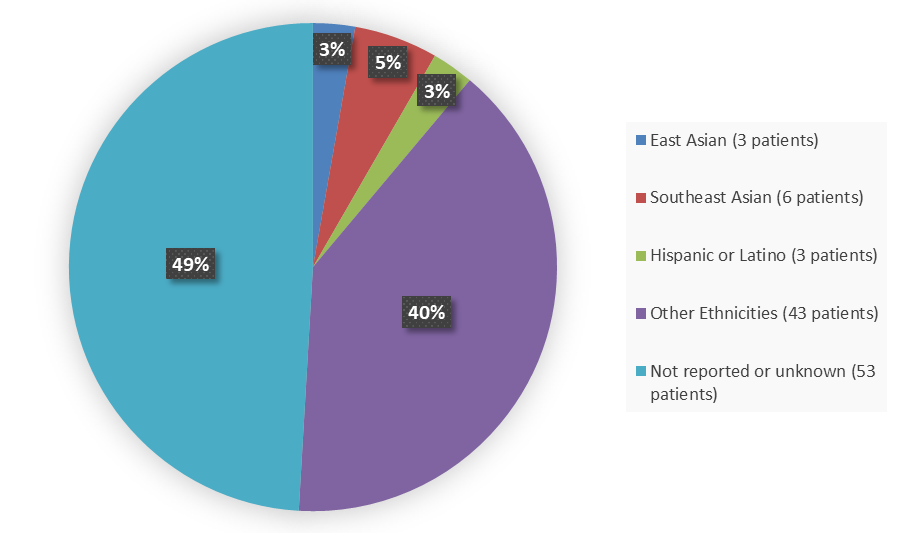
Figure 4 summarizes how many patients by ethnicity were in the combined trials used to evaluate the side effects of TRUSELTIQ.
Figure 4. Baseline Demographics by Ethnicity (Safety and Efficacy Population)
Source: Adapted from FDA Review
How were the trials designed?
There was one trial that provided data for TRUSELTIQ approval. The trial enrolled adult patients with bile duct cancer who had been treated previously with chemotherapy for their advanced cancer and whose tumors had a certain type of abnormality in the FGFR2 gene.
Patients received TRUSELTIQ once daily by mouth for 21 consecutive days followed by 7 days off therapy. This 28-day cycle was administered until disease progression or the side effects became too toxic. The trial measured the percentage of patients who achieved partial or complete shrinkage of their cancer and how long that shrinkage lasted (duration of response or DoR).
How were the trials designed?
There was one multi-center, open-label, single arm trial that provided data for approval of TRUSELTIQ.
Enrolled patients were required to have locally advanced unresectable or metastatic cholangiocarcinoma whose disease had progressed on or after at least one prior therapy, and an FGFR2 gene fusion or other rearrangement.
Patients received TRUSELTIQ in 28-day cycles at a dosage of 125 mg orally once daily for 21 consecutive days, followed by 7 days off therapy. TRUSELTIQ was administered until disease progression or unacceptable toxicity.
The major efficacy outcome measures were overall response rate (ORR) and duration of response (DoR) as determined by an independent review committee (IRC) according to RECIST v1.1.
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.