Drug Trials Snapshots: ZYNLONTA
HOW TO USE THIS SNAPSHOT
The information provided in Snapshots highlights who participated in the key clinical trials that supported the original FDA approval of this drug, and whether there were differences among sex, race, age, and ethnic groups. The “MORE INFO” bar shows more detailed, technical content for each section. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of the drugs.
LIMITATIONS OF THIS SNAPSHOT
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your healthcare provider about the benefits and risks of a drug.
Some of the information in this Snapshot is for presentation purposes and does not represent the approved conditions of use of this drug. Refer to the ZYNLONTA Prescribing Information for all of the approved conditions of use of this drug (e.g., indication(s), population(s), dosing regimen(s), safety information).
Snapshots are limited to the information available at the time of the original approval of the drug and do not provide information on who participated in clinical trials that supported later approvals for additional uses of the drug (if applicable).
ZYNLONTA (loncastuximab tesirine-lpyl)
(Zin lon’ tah)
ADC Therapeutics SA
Approval date: April 23, 2021
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
ZYNLONTA is used to treat adults with certain types of large B-cell lymphoma whose disease has come back or has not improved after at least two previous treatments. The types of lymphoma include diffuse large B-cell lymphoma (DLBCL), DLBCL arising from a low-grade lymphoma, and high-grade B-cell lymphoma.
Large B-cell lymphomas are fast-growing cancers of the lymph system, which is part of the body’s immune system.
How is this drug used?
ZYNLONTA is given by a healthcare provider directly into the bloodstream through a needle in the vein. This is known as an intravenous, or IV infusion. ZYNLONTA is given every 21 days (every 3 weeks).
Who participated in the clinical trials?
The FDA approved ZYNLONTA based on evidence from clinical trial ADCT-402-201 (LOTIS-2) that included 145 adult patients with large B-cell lymphoma who received at least two prior treatments that did not work or was no longer working.
Trials were conducted at 28 sites in the United States, United Kingdom, Italy, and Switzerland.
What are the benefits of this drug?
In a clinical trial with 145 patients with large B-cell lymphoma, 48% had a complete or partial shrinkage of their tumors (response).
ZYNLONTA was approved under FDA’s accelerated approval program, which provides earlier patient access to a promising new drug while the company continues to conduct clinical trials to confirm that the drug works well.
What are the benefits of this drug (results of trials used to assess efficacy)?
Table 1 below summarizes efficacy results established by the Overall Response Rate.
Table 1. Overall Response Rate and Duration of Response in Patients With Large B-Cell Lymphoma
| Outcome Per IRC | ZYNLONTA N=145 |
|---|---|
| Overall response rate, n (%)a | 48% |
| 95% CI | 40%, 57% |
| Complete response, n (%) | 24% |
| Partial response, n (%) | 24% |
| Duration of overall response | |
| Median (95% CI)b, months | 10.3 (6.9, NE) |
Source: ZYNLONTA Prescribing Information
a Per IRC using Lugano 2014 criteria
b Based on Kaplan-Meier estimation. Of 70 patients with objective response, 25 (36%) were censored prior to 3 months. Twenty-six percent of responders had a duration of response ≥6 months.
Abbreviations: CI, confidence interval; IRC, Independent Review Committee; NE, not estimable
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
- Sex: ZYNLONTA worked similarly in male and female patients.
- Race: The number of the patients of races other than White was small; therefore, differences in how well the drug worked among races could not be determined.
- Age: ZYNLONTA worked similarly among patients younger and older than 65 years of age.
Were there any differences in how well the drug worked in clinical trials among sex, race, and age groups?
Table 2 below summarizes efficacy results by sex, age, and race. Because of the small sample sizes, these exploratory analyses should be interpreted with caution.
Table 2. Subgroup Analyses of Overall Response Rate
| Subgroup | Total | Overall Response Rate n (%) |
|---|---|---|
| Sex | ||
| Male | 60 | 30 (50) |
| Female | 85 | 40 (47) |
| Age, years | ||
| <65 | 65 | 32 (49) |
| ≥65 | 80 | 38 (47) |
| Race | ||
| White | 130 | 62 (48) |
| Other* | 15 | 8 (53) |
Source: Adapted from FDA Review
* Other includes Black or African American (n=5), Asian (n=3), American Indian or Alaskan Native (n=1), Native Hawaiian or Pacific Islander (n=1), and Other (n=5).
What are the possible side effects?
ZYNLONTA may cause serious side effects including fluid retention, low blood cell counts (infection-fighting white blood cells, red blood cells, and platelets), infections, severe skin reactions, and harm to an unborn baby.
The most common side effects of ZYNLONTA are low levels of blood platelets (thrombocytopenia), abnormal liver blood tests, decreased level of infection-fighting white blood cells (neutropenia), low levels of red blood cells (anemia), increased blood sugar (hyperglycemia), fatigue, decreased levels of albumin, rash, fluid retention (edema), nausea, and musculoskeletal pain.
What are the possible side effects (results of trials used to assess safety)?
Table 3 and Table 4 below summarize adverse reactions in 145 adult patients with large B-cell lymphoma who received ZYNLONTA in the clinical trial.
Table 3. Adverse Reactions in ≥10% of Patients With Large B-Cell Lymphoma Who Received ZYNLONTA
| Adverse Reactions | ZYNLONTA N=145 |
|
|---|---|---|
| All Grades (%) | Grade 3 or 4a (%) | |
| General disorders and administration site conditions | ||
| Fatigueb | 38 | 1a |
| Edemac | 28 | 3a |
| Skin and subcutaneous tissue disorders | ||
| Rashd | 30 | 2a |
| Pruritus | 12 | 0 |
| Photosensitivity reaction | 10 | 2a |
| Gastrointestinal disorders | ||
| Nausea | 23 | 0 |
| Diarrhea | 17 | 2a |
| Abdominal paine | 14 | 3 |
| Vomiting | 13 | 0 |
| Constipation | 12 | 0 |
| Musculoskeletal and connective tissue disorders | ||
| Musculoskeletal painf | 23 | 1a |
| Metabolism and nutrition disorders | ||
| Decreased appetite | 15 | 0 |
| Respiratory disorders | ||
| Dyspneag | 13 | 1a |
| Pleural effusion | 10 | 2a |
| Infection | ||
| Upper respiratory tract infectionh | 10 | <1a |
Source: Adapted from FDA Review
a No Grade 4 adverse reactions occurred
b Fatigue includes fatigue, asthenia, and lethargy
c Edema includes edema, face edema, generalized edema, peripheral edema, ascites, fluid overload, peripheral swelling, swelling, and swelling face
d Rash includes rash, rash erythematous, rash maculopapular, rash pruritic, rash pustular, erythema, generalized erythema, dermatitis, dermatitis acneiform, dermatitis bullous, dermatitis exfoliative generalized, and palmar-plantar erythrodysesthesia syndrome
e Abdominal pain includes abdominal pain, abdominal discomfort, abdominal pain lower, and abdominal pain upper
f Musculoskeletal pain includes musculoskeletal pain, musculoskeletal chest pain, musculoskeletal discomfort, back pain, limb discomfort, myalgia, neck pain, non-cardiac chest pain, and pain in extremity
g Dyspnea includes dyspnea, and dyspnea exertional
h Upper respiratory tract infection includes upper respiratory tract infection, upper respiratory tract congestion, nasopharyngitis, rhinitis, rhinovirus infection, and sinusitis
Table 4. Select Laboratory Adverse Reactions in ≥10% of Patients With Large B-Cell Lymphoma Who Received ZYNLONTA
| Laboratory Abnormality | ZYNLONTAa N=145 |
|
|---|---|---|
| All Grades (%) | Grade 3 or 4b (%) | |
| Hematologic | ||
| Platelets decreased | 58 | 17 |
| Neutrophils decreased | 52 | 30 |
| Hemoglobin decreased | 51 | 10b |
| Chemistry | ||
| Gamma-glutamyl transferase increased | 57 | 21 |
| Glucose increased | 48 | 8 |
| Aspartate aminotransferase increased | 41 | <1b |
| Albumin decreased | 37 | <1b |
| Alanine aminotransferase increased | 34 | 3 |
Source: ZYNLONTA Prescribing Information
a The denominator used to calculate the rate varied from 143 to 145 patients based on the number of patients with a baseline laboratory value and at least one post-treated value
b No Grade 4 adverse reactions occurred
Were there any differences in side effects among sex, race and age?
- Sex: The occurrence of side effects was generally similar in male and female patients. However, males reported more serious adverse reactions, infections, and edema than females.
- Race: The number of the patients of races other than White was small; therefore, differences in the occurrence of side effects among races could not be determined.
- Age: The occurrence of side effects was generally similar in patients younger and older than 65 years of age.
Were there any differences in side effects of the clinical trials among sex, race, and age groups?
Table 5 below summarizes selected adverse reactions by sex and age subgroups. Because of the small sample sizes, these exploratory analyses should be interpreted with caution.
Table 5. Subgroup Analysis of Select Adverse Reactions by Sex and Age
| Adverse Reaction | Sex | Age | ||
|---|---|---|---|---|
| Male N=60 n (%) |
Female N=85 n (%) |
<65 Years N=65 n (%) |
≥65 Years N=80 n (%) |
|
| Serious adverse reaction | 30 (50) | 11 (13) | 26 (40) | 31 (39) |
| Infection | 27 (45) | 21 (25) | 18 (28) | 30 (37) |
| Edema | 20 (33) | 20 (23) | 17 (26) | 23 (29) |
Source: Adapted from FDA Review
DEMOGRAPHICS SNAPSHOT
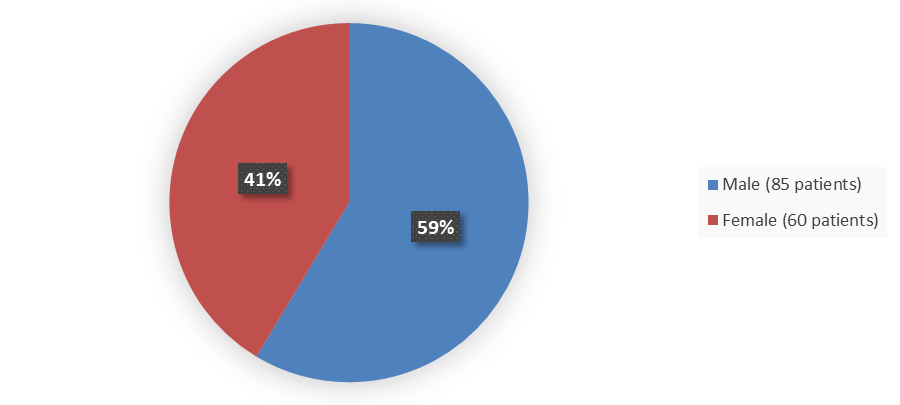
Figure 1 summarizes how many male and female patients were enrolled in the clinical trial used to evaluate the efficacy and safety of ZYNLONTA.
Figure 1. Baseline Demographics by Sex
Source: Adapted from FDA review
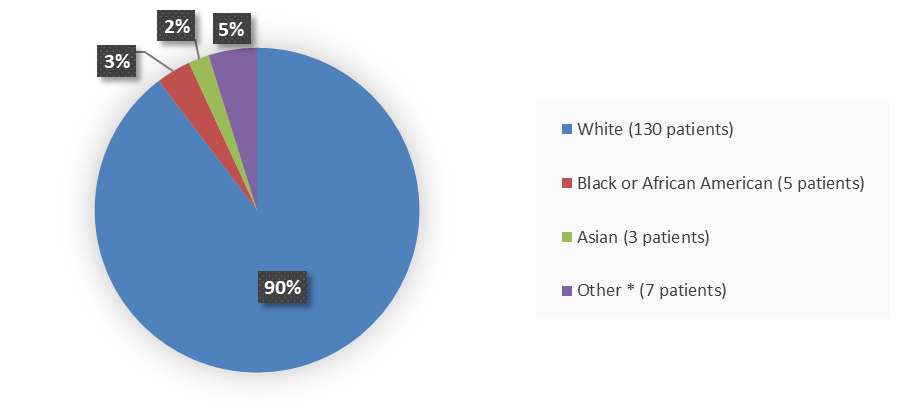
Figure 2 summarizes the percentage of patients by race enrolled in the clinical trial used to evaluate the efficacy and safety of ZYNLONTA.
Figure 2. Baseline Demographics by Race
Source: Adapted from FDA review
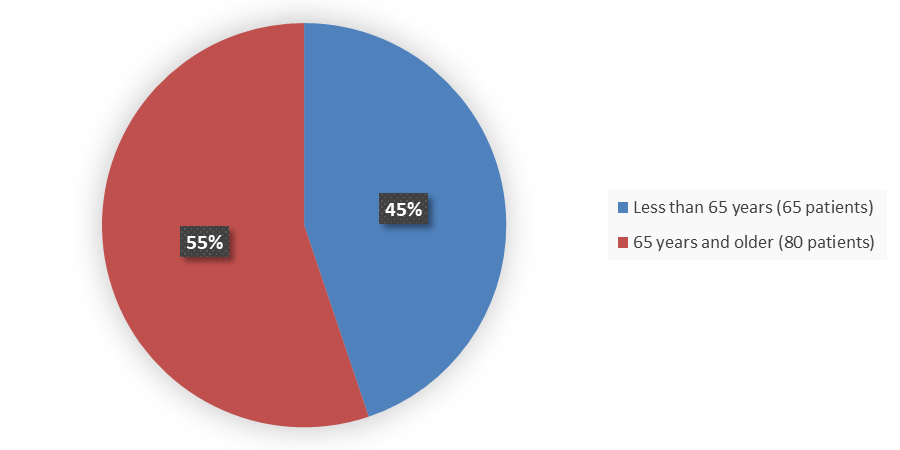
Figure 3 summarizes the percentage of patients by age enrolled in the clinical trial used to evaluate the efficacy and safety of ZYNLONTA
Figure 3. Baseline Demographics by Age
Source: Adapted from FDA Review
Who participated in the trials?
Table 6 below summarizes the demographics of the safety and efficacy population.
Table 6. Baseline Demographics of the Efficacy and Safety Population
| Demographic Characteristics | ZYNLONTA N=145 n (%) |
|---|---|
| Age, years | |
| Mean (SD) | 63 (13.6) |
| Median (minimum, maximum) | 66 (23, 94) |
| Age categories, years, n (%) | |
| <65 | 65 (45) |
| ≥65 | 80 (55) |
| Sex, n (%) | |
| Male | 85 (59) |
| Female | 60 (41) |
| Race, n (%) | |
| White | 130 (90) |
| Black or African American | 5 (3.4) |
| Asian | 3 (2.1) |
| Othera | 7 (4.8) |
| Region, n (%) | |
| US | 59 (41) |
| Non-US | 86 (59) |
| Body mass index (kg/m2) | |
| Mean (SD) | 26.9 (5.7) |
| Median (minimum, maximum) | 25.9 (17.2, 50.5) |
| ECOG performance status, n (%) | |
| 0 | 58 (40) |
| 1 | 78 (54) |
| 2 | 9 (6) |
| Histology, n (%) | |
| DLBCL, NOS | 127 (88) |
| DLBCL arising from low grade lymphoma | 29 (20) |
| High-grade B-cell lymphoma | 11 (8) |
| Stage of disease at screening, n (%) | |
| I | 10 (7) |
| II | 23 (16) |
| III | 19 (13) |
| IV | 93 (64) |
| Prior systemic therapy, n (%) | |
| Median (minimum, maximum) | 3.0 (2, 7) |
| 2 | 63 (43) |
| 3 | 35 (24) |
| ≥3 | 47 (32) |
| Response to last treated, n (%) | |
| Refractory | 92 (63) |
| Relapsed | 43 (30) |
| Other | 10 (7) |
| Prior stem cell transplant, n (%) | 24 (17) |
| Prior CAR T-cell therapy, n (%) | 13 (9) |
Source: Adapted from FDA review
a Other includes American Indian or Alaska Native, Native Hawaiian or Pacific Islander, and Other Abbreviations: CAR T-cell, Chimeric antigen receptor T-cell; DLBCL, diffuse large B-cell lymphoma; ECOG, Eastern Cooperative Oncology Group; NOS, not otherwise specified; SD, standard deviation; US, United States
How were the trials designed?
The benefit and side effects of ZYNLONTA were evaluated in one clinical trial, ADCT-402-201 (LOTIS-2), that included adult patients with large B-cell lymphoma. The clinical trial enrolled adult patients with large B-cell lymphoma after at least two prior treatments that did not work or were no longer working. Patients received ZYNLONTA 0.15 mg/kg every 3 weeks for 2 treatment cycles, then 0.075 mg/kg every 3 weeks for subsequent treatment cycles. ZYNLONTA treatment was continued until either disease worsened or patients experienced unacceptable side effects (toxicity). The benefit of ZYNLONTA was evaluated by measuring how many patients had complete or partial tumor shrinkage (response) and by how long that response lasted. Patients in the clinical trial were also evaluated for side effects for the purpose of this drug application.
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.