Drug Trials Snapshots: SUNLENCA
HOW TO USE THIS SNAPSHOT
The information provided in Snapshots highlights who participated in the key clinical trials that supported the original FDA approval of this drug, and whether there were differences among sex, race, age, and ethnic groups. The “MORE INFO” bar shows more detailed, technical content for each section. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of the drugs.
LIMITATIONS OF THIS SNAPSHOT:
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your healthcare provider about the benefits and risks of a drug.
Some of the information in this Snapshot is for presentation purposes and does not represent the approved conditions of use of this drug. Refer to the SUNLENCA Prescribing Information for all of the approved conditions of use of this drug (e.g., indication(s), population(s), dosing regimen(s), safety information).
Snapshots are limited to the information available at the time of the original approval of the drug and do not provide information on who participated in clinical trials that supported later approvals for additional uses of the drug (if applicable).
SUNLENCA (lenacapavir)
sun-LEN-kuh
Gilead Sciences, Inc.
Original Approval date: December 22, 2022
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
SUNLENCA is a drug that is used with other drugs for the treatment of human immunodeficiency virus-1 (HIV-1) infection in adults who:
- have received several antiretroviral (anti-HIV-1) drugs in the past, and
- have HIV-1 virus that is not responding to many antiretroviral medicines, and
- who are failing their current antiretroviral therapy.
How is this drug used?
SUNLENCA is used in combination with other antiretroviral medications approved for the treatment of HIV-1 infection.
SUNLENCA is a tablet that is taken by mouth and by injections that are given to patients by their healthcare provider under the skin (subcutaneous injection) in the stomach-area (abdomen). SUNLENCA oral tablets and injections are used together when a patient first starts treatment with SUNLENCA. After starting SUNLENCA with oral tablets and injections, patients receive two SUNLENCA injections every 6 months.
Who participated in the clinical trials?
The FDA approved SUNLENCA based on evidence of efficacy and safety from a clinical trial of 72 patients with HIV-1 infection. The trial was conducted at 31 sites in 11 countries including the United States.
How were the trials designed?
The benefits and side effects of SUNLENCA were evaluated in one clinical trial that enrolled patients with HIV-1 infection who did not respond to various anti-HIV treatments anymore.
For the evaluation of efficacy, the trial consisted of one group (cohort), Group 1. Group 1 patients received 14 days of oral SUNLENCA or placebo in addition to their failing antiretroviral regimen (“functional monotherapy period”). The benefit of SUNLENCA was assessed by measuring the number of HIV-1 copies in the blood from the beginning to the end of the 14-day “functional monotherapy period.”
For the evaluation of side effects, the trial consisted of two groups (cohorts), Group 1 and Group 2, and were followed for approximately 52 weeks.
How were the trials designed?
The trial was composed of two cohorts. Subjects were enrolled into the randomized cohort (Group 1, N=36) if they had a <0.5 log10 HIV-1 RNA decline compared to the screening visit. Subjects were enrolled into the non-randomized cohort (Group 2, N=36) if they had a ≥0.5 log10 HIV-1 RNA decline compared to the screening visit or after Group 1 reached its planned sample size.
In the 14-day functional monotherapy period, subjects in Group 1 were randomized in a 2:1 ratio in a blinded fashion to receive either SUNLENCA or placebo, while continuing their failing regimen. This period was to establish the virologic activity of SUNLENCA. After the functional monotherapy period, subjects who had received SUNLENCA continued on SUNLENCA along with an optimized background regimen and subjects who had received placebo initiated SUNLENCA along with an optimized background regimen. Subjects in Group 2 initiated SUNLENCA and an optimized background regimen on Day 1.
The primary efficacy endpoint was the proportion of subjects in Group 1 who achieved ≥0.5 log10 copies/mL reduction from baseline in HIV-1 RNA at the end of the functional monotherapy period.
DEMOGRAPHICS SNAPSHOT
In the clinical trial, the efficacy of SUNLENCA was assessed in 36 patients (Group 1), and side effects were assessed in 72 patients (Group 1 and Group 2).
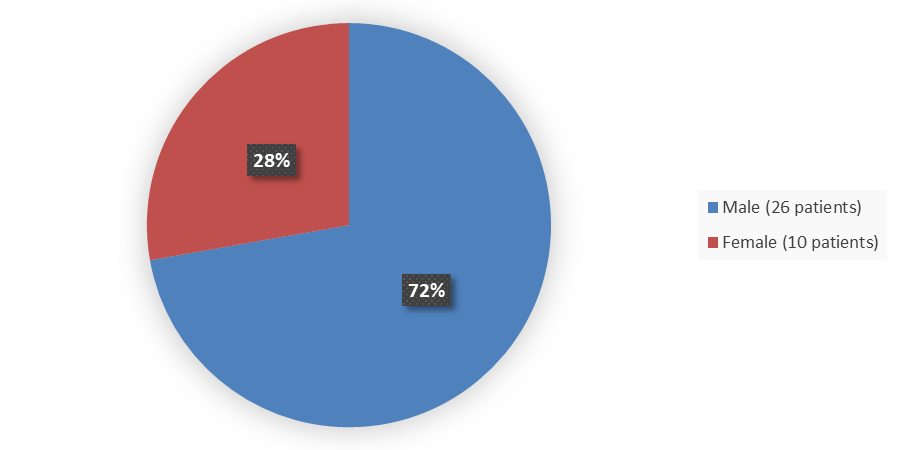
Figure 1. Baseline Demographics by Sex (Efficacy population)
Source: Adapted from FDA Review
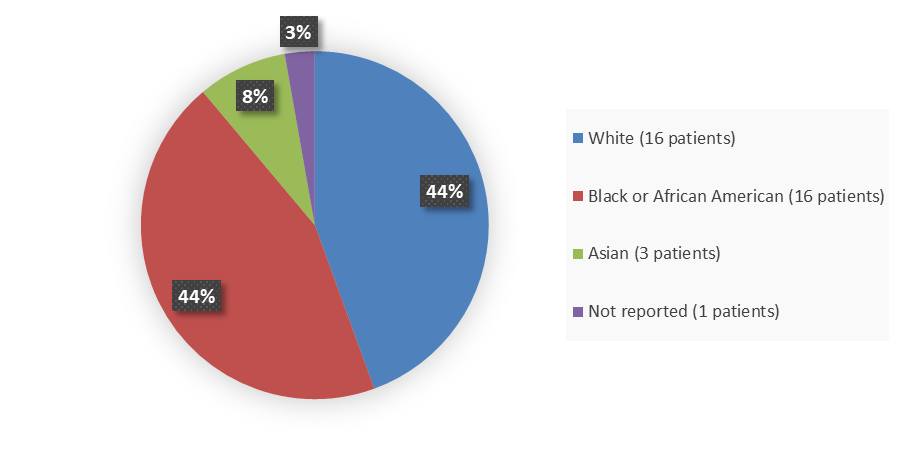
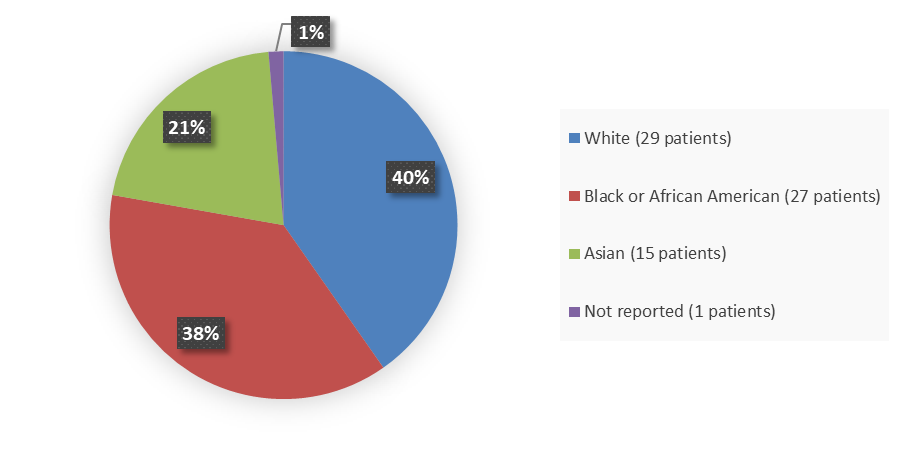
Figure 2. Baseline Demographics by Race (Efficacy Population)
Source: Adapted from FDA Review
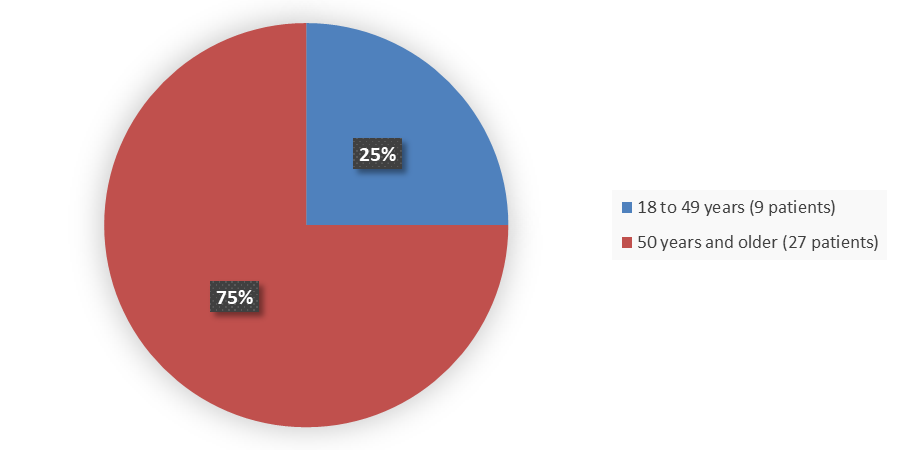
Figure 3. Baseline Demographics by Age (Efficacy Population)
Source: Adapted from FDA Review
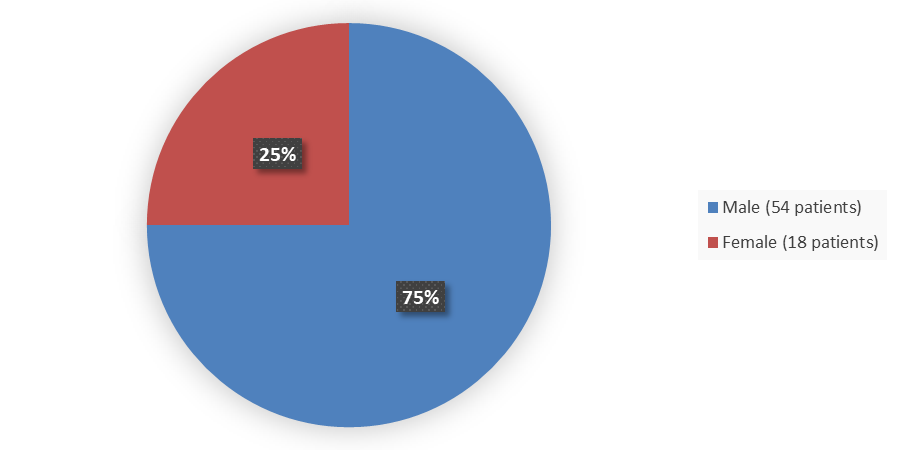
Figure 4. Baseline Demographics by Sex (Safety Population)
Source: Adapted from FDA Review
Figure 5. Baseline Demographics by Race (Safety Population)
Source: Adapted from FDA Review
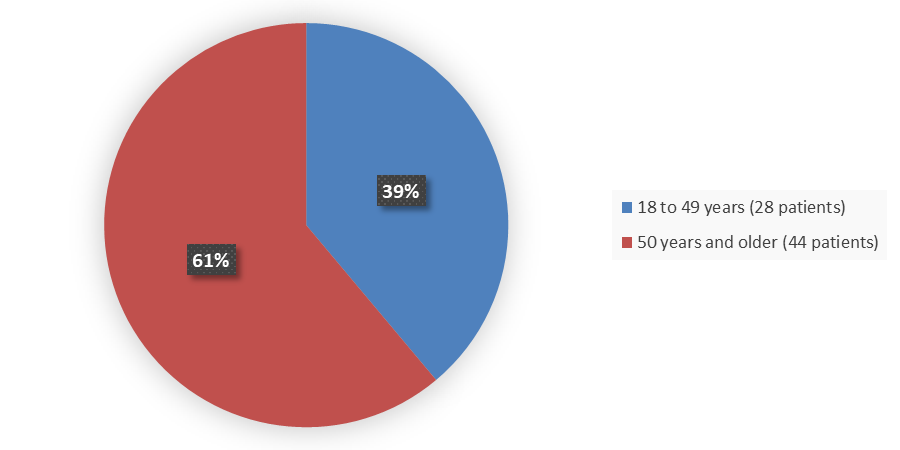
Figure 6. Baseline Demographics by Age (Safety Population)
Source: Adapted from FDA Review
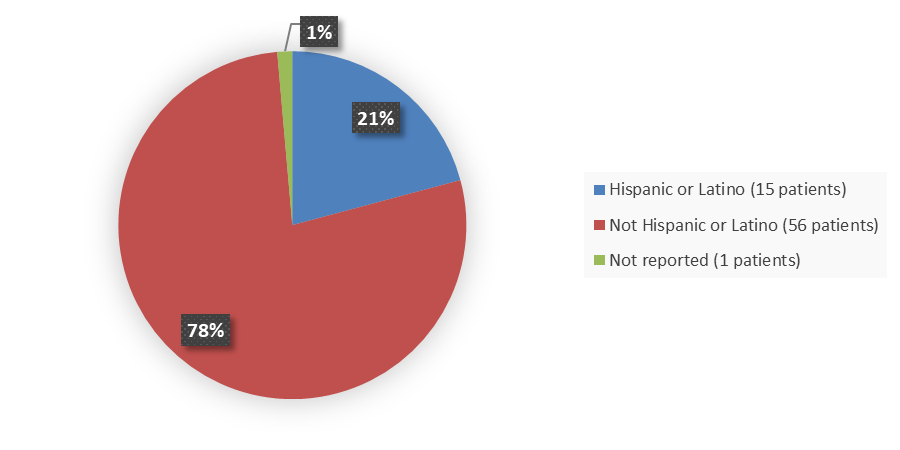
Figure 7. Baseline Demographics by Ethnicity (Safety Population)
Source: Adapted from FDA Review
Who participated in the trials?
The demographics from the clinical trial of 72 adults with HIV-1 infection is presented in Table 4.
Table 4. Trial Demographics
|
Demographic Parameter |
Number of Patients |
|---|---|
|
Sex |
|
|
Male |
54 (75.0) |
|
Female |
18 (25.0) |
|
Race |
|
|
White |
29 (40.3) |
|
Black |
27 (37.5) |
|
Asian |
15 (20.8) |
|
Not reported |
1 (1.4) |
|
Ethnicity |
|
|
Not Hispanic |
56 (77.8) |
|
Hispanic |
15 (20.8) |
|
Not reported |
1 (1.4) |
|
Age, years |
|
|
Mean |
50 |
|
Median |
52 |
|
Minimum, maximum |
23, 78 |
|
Age group, years |
|
|
≥18 to 49 |
28 (38.9) |
|
≥50 |
44 (61.1) |
|
≥65 |
6 (8.3) |
|
Region |
|
|
United States |
42 (58.3) |
|
Outside United States |
30 (41.7) |
Source: Adapted from FDA Review
What are the benefits of this drug?
SUNLENCA reduced viral load of HIV-1. In the trial, 21 of the 24 patients (88%) treated with SUNLENCA experienced a significant decrease in their HIV-1 RNA levels 2 weeks after SUNENCA was added to their failing antiretroviral regimens.
After 52 weeks of SUNLENCA treatment (in combination with other antiretroviral drugs), 83% of the patients achieved HIV-1 RNA suppression.
What are the benefits of this drug (results of trials used to assess efficacy)?
Table 1 summarizes the primary efficacy endpoint, which was the proportion of patients in the randomized group who received SUNLENCA versus placebo who had achieved a decline in viral load at the end of the 14-day “functional monotherapy period” greater than or equal to a 0.5 log10.
Table 1. Proportion of Subjects Achieving a ≥0.5 log10 Decrease in HIV-1 Viral Load at the End of the Functional Monotherapy Period in the CAPELLA Trial (Group 1)
|
|
SUNLENCA |
Placebo |
|---|---|---|
|
Proportion of subjects achieving a ≥0.5 log10 decrease in viral load |
87.5% |
16.7% |
|
Treatment difference (95% CI) |
70.8% (34.9 to 90.0)* |
|
Source: SUNLENCA Prescribing Information
* p<0.0001
Abbreviations: CI, confidence interval
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
- Sex: SUNLENCA worked better in male patients than female patients.
- Race: SUNLENCA worked similar in White and Black or African American patients. The number of patients of other races was small; therefore, differences in how SUNLENCA worked involving other races could not be determined.
- Age: SUNLENCA worked better in patients below 65 years of age than above 65 years of age.
Were there any differences in how well the drug worked in clinical trials among sex, race, and age groups?
Table 2 summarizes the subgroup analysis by demographic group for the primary efficacy endpoint. This analysis is limited by the small sample size.
Table 2. Percentage of Patients Achieving a ≥0.5 log10 Decrease at the End of the Functional Monotherapy Period
|
Subgroup |
SUNLENCA |
Placebo |
|---|---|---|
|
Sex |
||
|
Male |
94.1 (16/17) |
11.1 (1/9) |
|
Female |
71.4 (5/7) |
33.3 (1/3) |
|
Race |
||
|
Black/African American |
90.0 (9/10) |
16.7 (1/6) |
|
White |
91.7 (11/12) |
25.0 (1/4) |
|
Asian |
50.0%(1/2) |
0% (0/1) |
|
Not reported |
0 |
0% (0/1) |
|
Age, years |
||
|
<50 |
83.3 (5/6) |
0 (0/3) |
|
≥50 |
88.9 (16/18) |
22.2 (2/9) |
|
Ethnicity |
||
|
Hispanic |
100 (6/6) |
50 (2/4) |
|
Not Hispanic |
83 (15/18) |
0 (0/7) |
|
Not permitted |
0 |
0 (0/1) |
Source: Adapted from FDA Review
What are the possible side effects?
SUNLENCA may cause serious side effects including changes in the immune system (immune reconstitution syndrome) and injection site reactions. During the initial phase of treatment with SUNLENCA, the immune system may get stronger and begin to fight infections that were hidden, a condition called immune reconstitution syndrome. Immune reconstitution syndrome is a serious condition. In addition, SUNLENCA can cause injection site reactions, including nodules and indurations at the injection site. Some injection site nodules and indurations may take longer to resolve than other injection site reactions, and in some patients nodules and indurations did not fully resolve.
Other than injection site reactions, the most common side effect associated with SUNLENCA was nausea.
What are the possible side effects (results of trials used to assess safety)?
Injection site reactions. Of the 72 subjects in the trial who were used to assess safety, 65% experienced an injection site reaction attributed to SUNENCA through at least the Week 52 visit. The injection site reactions reported in more than 1% of subjects were swelling (36%), pain (31%), erythema (31%), nodule (25%), induration (15%), pruritus (6%), extravasation (3%) and mass (3%). Injection site reactions reported in 1% of subjects included discomfort, hematoma, edema, and ulcer.
Injection site nodules and injection site indurations at the injection site took longer to resolve than other injection site reactions. The median time to resolution of all injection site reactions, excluding nodules and indurations, was 5 days (range: 1 to 183). The median time to resolution of nodules and indurations associated with the first injections of SUNLENCA was 148 (range: 41 to 727) and 70 (range: 3 to 252) days, respectively. After a median follow up of 553 days, 30% of nodules and 13% of indurations (in 10% and 1% of subjects, respectively) associated with the first injections of SUNLENCA had not fully resolved.
Were there any differences in side effects of the clinical trials among sex, race, and age?
- Sex: The occurrence of side effects was similar in both female and male patients.
- Race: The occurrence of side effects was similar in White, Asian and Black or African American patients.
- Age: The occurrence of side effects was similar to patients in patients below and above 65 years of age.
Were there any differences in side effects of the clinical trials among sex, race, and age groups?
Table 3 summarizes adverse reactions occurring in two or more patients by sex, age, race, and ethnicity. This analysis is limited by the small sample size.
Table 3. Subgroup Analysis of Adverse Reactions
|
|
N |
Adverse Reaction, n (%) |
|
|---|---|---|---|
|
Injection Site Reaction |
Nausea |
||
|
Overall |
72 |
47 (65) |
3 (4) |
|
Sex |
|||
|
Male |
54 |
36 (67) |
2 (4) |
|
Female |
18 |
11 (61) |
1 (6) |
|
Age, years |
|||
|
<50 |
28 |
19 (64) |
0 |
|
≥50 |
44 |
28 (64) |
3 (7) |
|
Race |
|||
|
White |
29 |
19 (66) |
1 (3) |
|
Black or African American |
27 |
14 (52) |
2 (7) |
|
Asian |
15 |
13 (87) |
0 |
|
Not reported |
1 |
1 (100) |
0 |
|
Ethnicity |
|||
|
Hispanic |
15 |
9 (60) |
0 |
|
Not Hispanic |
56 |
37 (66) |
3 (5) |
|
Not reported |
1 |
1 (100) |
0 |
Source: Adapted from FDA Review
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.