FY2015 Regulatory Science Research Report: Physiologically-Based Absorption and Pharmacokinetic Models
Introduction
Physiologically-based absorption and pharmacokinetic (PBAPK) modeling and simulation is a core technology for the future of generic drug products. These types of models integrate device performance and formulation factors with physiologic parameters (traditional PBPK) to predict in vivo performance of drug products. The PBAPK models are tools for knowledge and risk management as they contain our best scientific understanding of the interaction between products and physiology. They allow us to evaluate in vivo performance of drug products in situations where regular BE studies are not conducted, such as special populations, local concentrations, a wide range of formulation candidates, and formulation strengths with BE waivers. The future of generic drugs involves industry use of PBAPK models to formulate drugs and FDA use to evaluate and optimize BE standards.
Research
Prior to 2014, OGD had established expertise in using PBAPK models for orally administered drug products and implemented the technology in various regulatory activities, including consultation responses, citizen’s petition responses, BE recommendations for specific drug products, and ANDA reviews. The impact on regulatory decision making is summarized in the article “Modeling and Simulation of Biopharmaceutical Performance” in Clinical Pharmacology & Therapeutics in 2014. PBAPK played an important role in responding to safety consultation requests on mixed amphetamine salts extended release capsules, dextroamphetamine sulfate extended release capsules, and methylphenidate extended-release tablets.
To improve PBAPK modeling for oral products, our research project with the University of Michigan models GI fluid hydrodynamics; samples GI tract fluids composition and pH; tests novel dissolution methods; and uses in vivo PK studies to calibrate the model and verify model predictions. This project will benefit all oral products, including locally-acting GI drug products.
In 2014, research in the area of PBAPK modeling was expanded to non-oral drug products. A Request for Application (RFA-FD-14-012: Physiologically Based Absorption and Pharmacokinetic Modeling and Simulation for Non-gastrointestinally Absorbed Drug Products in Humans (U01)) was posted on March 20, 2014. Twenty-four proposals were received in response to the RFA; seven were funded. The funded grants cover absorption via skin, eye, nose, and lung as well as PBPK models for liposomes. The ultimate outcomes of these grants are computational tools that bridge formulation in vitro performance and in vivo performance for these more complex routes of delivery.
Moving forward, collaborative efforts between ORS and external investigators will improve PBAPK tools for generic drug development and evaluation.
Figure 17. Impact of modeling and simulation
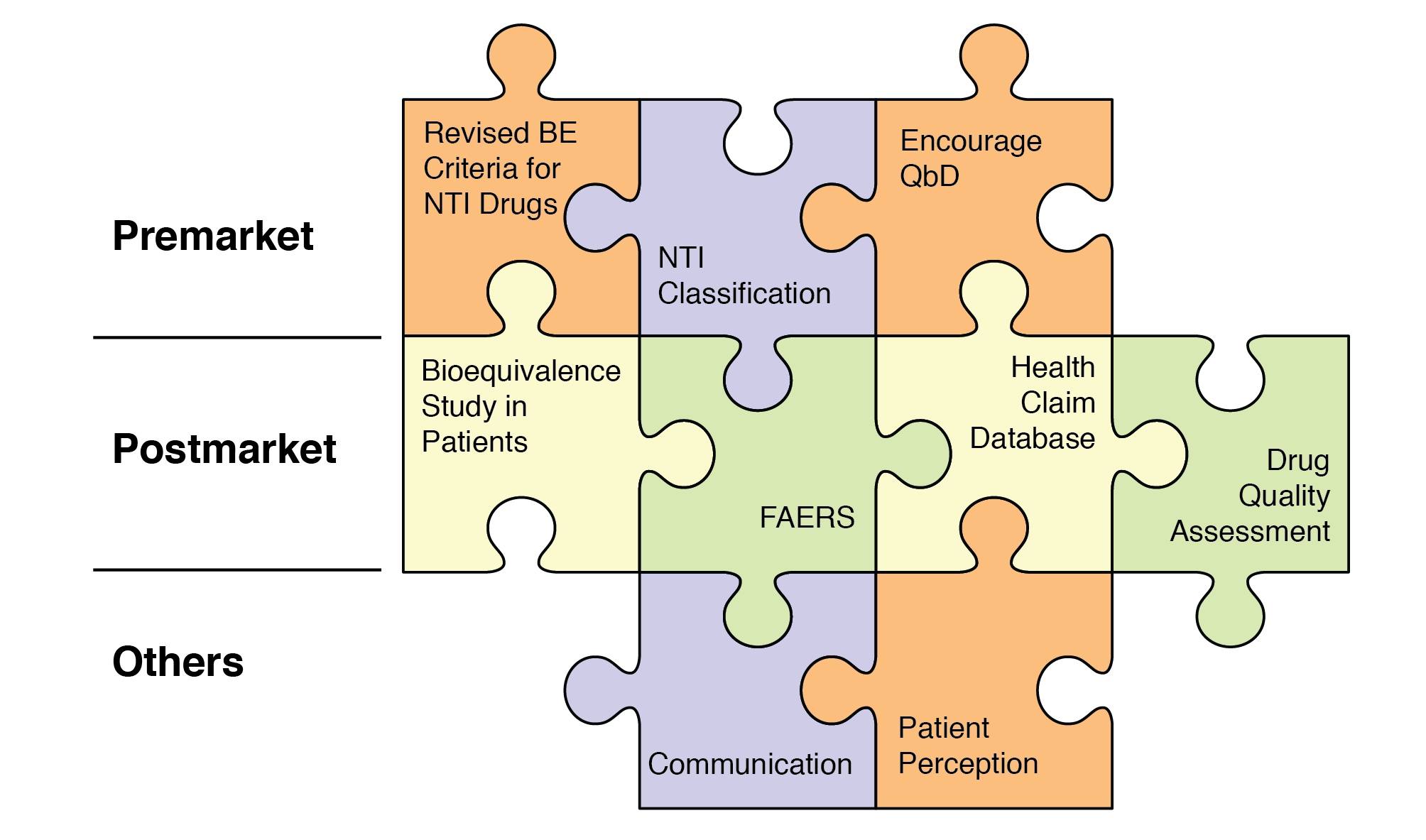
Source: Zhang X. Application of physiologically based pharmacokinetic (PBPK) modeling in generic drug evaluation. ORS presentation. (Mar 2015)
Figure 18. Application of physiologically-based pharmacokinetic (PBPK) modeling in generic drug evaluation
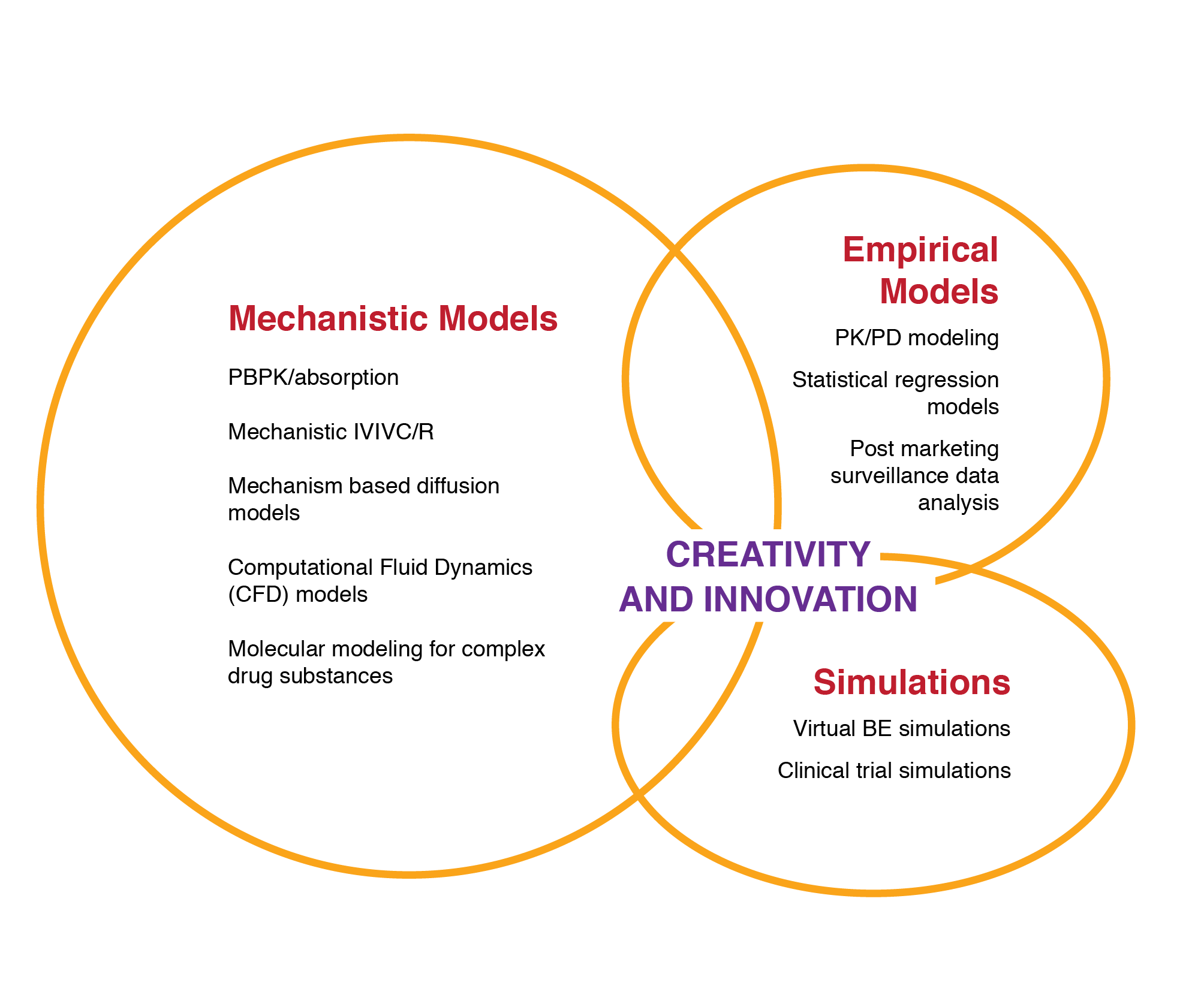
A broad spectrum of modeling and simulation is applied.
Source: Zhang X. Application of physiologically based pharmacokinetic (PBPK) modeling in generic drug evaluation. ORS presentation. (Mar 2015)
Figure 19. Modeling and simulation of biopharmaceutical performance
Source: Zhang X, Lionberger RA. Modeling and simulation of biopharmaceutical performance. Clin Pharmacol Ther 95(5):480-2 (May 2014)
ORS staff facilitating research in this area
- Xinyuan Zhang, Jianghong Fan, Edwin Chow, Andrew Babiskin, Hopi Lin, Eleftheria Tsakalozou, Arjang Talattof, Ross Walenga, and other ORS staff members
Projects and Collaborators
- Modernization of in vivo - in vitro oral bioperformance prediction and assessment. Gordon Amidon, University of Michigan
- Site PI: James Brasseur
- Contract #: HHSF223201310144C
- Physiologically based biopharmaceutics and pharmacokinetics of drug products for dermal absorption in humans
- Site PI: Michael Roberts
- Grant #: 1U01FD005232-01
- Development and validation of dermal PBPK modelling platform towards virtual bioequivalence assessment considering population variability
- Site PI: Sebastian Polak
- Grant #: 1U01FD005225-01
- PBPK modeling and simulation for ocular dosage forms
- Site PI: Michael B Bolger
- Grant #: 1U01FD005211-01
- An integrated multiscale-multiphysics modeling and simulation of ocular drug delivery with whole-body pharmacokinetic response
- Site PI: Kay Sun
- Grant #: 1U01FD005219-01
- Development of hybrid CFD-PBPK models for absorption of intranasal corticosteroids
- Site PI: Jeff Schroeter
- Grant #: 1U01FD005201-01
- A predictive multiscale computational tool for simulation of lung absorption and pharmacokinetics and optimization of pulmonary drug delivery
- Site PI: Peng Guo
- Grant #: 1U01FD005214-01
- Physiologically based pharmacokinetic model for drugs encapsulated into liposomes
- Site PI: Yanguang Cao
- Grant #: 1U01FD005206-01
Publications and Presentations
- Zhang X et al. Modeling and simulation of biopharmaceutical performance. Clin Pharmacol Ther 95(5):480-2. (May 2014)
- Babiskin et al. Application of physiologically based absorption modeling for amphetamine salts drug products in generic drug evaluation. J Pharm Sci (2015)
- Patel et al. Mechanistic modelling of dermal drug absorption using the Simcyp Multi-phase Multi-layer MechDermA model: Case study of a transdermal patch formulation of weak base drug timolol. Barrier Function of Mammalian Skin Gordon Research Conference: Defining, Investigating and Surmounting the Barrier. 2015 Waterville Valley, NH
- Polak et al. Towards mechanistic simulation and prediction of bioequivalence studies of topical formulations case study with two diclofenac formulations. Barrier Function of Mammalian Skin Gordon Research Conference: Defining, Investigating and Surmounting the Barrier. 2015 Waterville Valley, NH
- Cristea et al. Prediction of cutaneous PK profiles after topical application - a comparison between the novel MPML-MechDermA model and the current MechDermA model in Simcyp Simulator Case Study: Erythromycin. DMDG-100 Years of Drug Delivery, Ware, UK
- Merdy et al. Cellular Pharmacokinetic/pharmacodynamic of doxorubicin in a wide array of cell lines. 2015 American Association of Pharmaceutical Scientists (AAPS) Conference, Orlando, FL
- Kimbell et al. Simulating Nasal Spray Deposition: Effects of Spray Nozzle Presence in the Nasal Vestibule. International Society for Aerosols in Medicine meeting during, 2015 Munich, Germany
Outcomes
- Draft bioequivalence guidance for dextroamphetamine sulfate and dextroamphetamine hydrochloride extended release capsules
- Draft bioequivalence guidance for methylphenidate hydrochloride modified release products
