OST Super Office Reorganization Frequently Asked Questions (FAQs)
Effective immediately, the Office of Strategic Partnerships and Technology Innovation (OST) has been elevated to a Super Office within the Center. The Super Office Structure will increase organizational capabilities and advance our efforts to meet MDUFA V commitments, as well as our 2022-2025 Strategic Priorities.
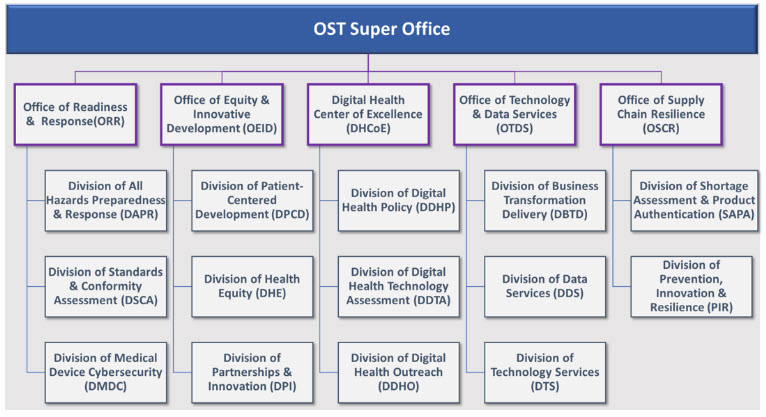
By elevating OST to a Super Office, several organizational shifts will occur, including reorganizing into five offices: the Office of Supply Chain Resilience (OSCR), Digital Health Center of Excellence (DHCoE), Office of Technology and Data Services (OTDS), Office of Readiness and Response (ORR), and Office of Equity and Innovative Development (OEID). For more information on the offices and their function, see the CDRH Statement.
Frequently Asked Questions
Below you will find answers to the most commonly asked questions about implementation of the OST reorganization.
- Why did OST reorganize?
- What is the benefit of the OST reorganization?
- Did my OST points of contact change?
- What were the changes to OST’s organizational structure?
- Where should I go if I have questions?
Why did OST reorganize?
OST has experienced growth since it was created in 2019 and the super office structure gives OST programmatic areas enhanced visibility, support, and critical resources to meet our expanded public health mission. This reorganization intends to better integrate the collective work across the OST to serve our customers’ best interest; achieve more efficient work processes; optimize decision‐making and ensure process and policy consistency for quality assurance.
What is the benefit of the OST reorganization?
There are many benefits to the new structure including but not limited to:
- Generating efficiencies – and opportunities – that allow OST to more fully realize our public health mission and vision. For example, we have CDRH and Agency priorities around health equity. Putting teams working on patient science and diverse patient populations in an office focused on health equity and innovation will enhance collaboration and communication. This in turn enables us to better integrate diverse patient perspectives into clinical study design so that barriers to diverse patient recruitment, participation, and retention are reduced which ultimately results in high quality, safe and effective devices for all patients.
- Elevating program functions in a way that reflects our desire to optimize decision-making and innovate with greater agility to respond to emerging needs – both externally and internally. For example, as an office DHCoE will have a higher profile which will support taking bold steps to embrace responsible innovation that protects patients, addresses bias, advances health equities, and supports a diverse and agile workforce.
- Strengthens the team-based approaches to our work and enables more communication and collaboration across OST, the Center, and the Agency, which have proven to be an essential ingredient to our success. For example, our programmatic work in medical device supply chain resiliency experienced growth during the last few years. This reorganization will bolster our ability to proactively monitor and communicate risks to stakeholders, perform data driven impact assessments, and inform regulatory mitigations to ensure the availability of safe and effective medical devices.
Did my OST points of contact change?
The reorganization did impact some points of contact within OST. Changes to contact information are available in CDRH's Management Directory and communicated through the offices.
What were the changes to OST’s organizational structure?
OST previously housed 3 divisions and 6 programs. The new structure which includes 5 offices and 14 divisions better aligns activities to better meet our public health mission.
Where should I go if I have questions?
Please send questions to the Division of Industry and Consumer Education (DICE@fda.hhs.gov) with the subject: “OST Reorg”.