3D imaging
The Amazing Brain: A Sharper Image of the Pyramidal Tract
Posted on by Dr. Francis Collins
Flip the image above upside down, and the shape may remind you of something. If you think it resembles a pyramid, then you and a lot of great neuroscientists are thinking alike. What you are viewing is a colorized, 3D reconstruction of a pyramidal tract, which are bundles of nerve fibers that originate from the brain’s cerebral cortex and relay signals to the brainstem or the spinal cord. These signals control many important activities, including the voluntary movement of our arms, legs, head, and face.
For a while now, it’s been possible to combine a specialized form of magnetic resonance imaging (MRI) with computer modeling tools to produce 3D reconstructions of complicated networks of nerve fibers, such as the pyramidal tract. Still, for technical reasons, the quality of these reconstructions has remained poor in parts of the brain where nerve fibers cross at angles of 40 degrees or less.
The video above demonstrates how adding a sophisticated algorithm, called Orientation Distribution Function (ODF)-Fingerprinting, to such modeling can help overcome this problem when reconstructing a pyramidal tract. It has potential to enhance the reliability of these 3D reconstructions as neurosurgeons begin to use them to plan out their surgeries to help ensure they are carried out with the utmost safety and precision.
In the first second of the video, you see gray, fuzzy images from a diffusion MRI of the pyramidal tract. But, very quickly, a more colorful, detailed 3D reconstruction begins to appear, swiftly filling in from the top down. Colors are used to indicate the primary orientations of the nerve fibers: left to right (red), back to front (green), and top to bottom (blue). The orange, magenta, and other colors represent combinations of these primary directional orientations.
About three seconds into the video, a rough draft of the 3D reconstruction is complete. The top of the pyramidal tract looks pretty good. However, looking lower down, you can see distortions in color and relatively poor resolution of the nerve fibers in the middle of the tract—exactly where the fibers cross each other at angles of less than 40 degrees. So, researchers tapped into the power of their new ODF-Fingerprinting software to improve the image—and, starting about nine seconds into the video, you can see an impressive final result.
The researchers who produced this amazing video are Patryk Filipiak and colleagues in the NIH-supported lab of Steven Baete, Center for Advanced Imaging Innovation and Research, New York University Grossman School of Medicine, New York. The work paired diffusion MRI data from the NIH Human Connectome Project with the ODF-Fingerprinting algorithm, which was created by Baete to incorporate additional MRI imaging data on the shape of nerve fibers to infer their directionality [1].
This innovative approach to imaging recently earned Baete’s team second place in the 2021 “Show Us Your BRAINs” Photo and Video contest, sponsored by the NIH-led Brain Research through Advancing Innovative Neurotechnologies® (BRAIN) Initiative. But researchers aren’t stopping there! They are continuing to refine ODF-Fingerprinting, with the aim of modeling the pyramidal tract in even higher resolution for use in devising new and better ways of helping people undergoing neurosurgery.
Reference:
[1] Fingerprinting Orientation Distribution Functions in diffusion MRI detects smaller crossing angles. Baete SH, Cloos MA, Lin YC, Placantonakis DG, Shepherd T, Boada FE. Neuroimage. 2019 Sep;198:231-241.
Links:
Brain Research through Advancing Innovative Neurotechnologies® (BRAIN) Initiative (NIH)
Human Connectome Project (University of Southern California, Los Angeles)
Steven Baete (Center for Advanced Imaging Innovation and Research, New York University Grossman School of Medicine, New York)
Show Us Your BRAINs! Photo and Video Contest (BRAIN Initiative/NIH)
NIH Support: National Institute of Biomedical Imaging and Bioengineering; National Institute of Neurological Disorders and Stroke; National Cancer Institute
Watching Cancer Cells Play Ball
Posted on by Dr. Francis Collins
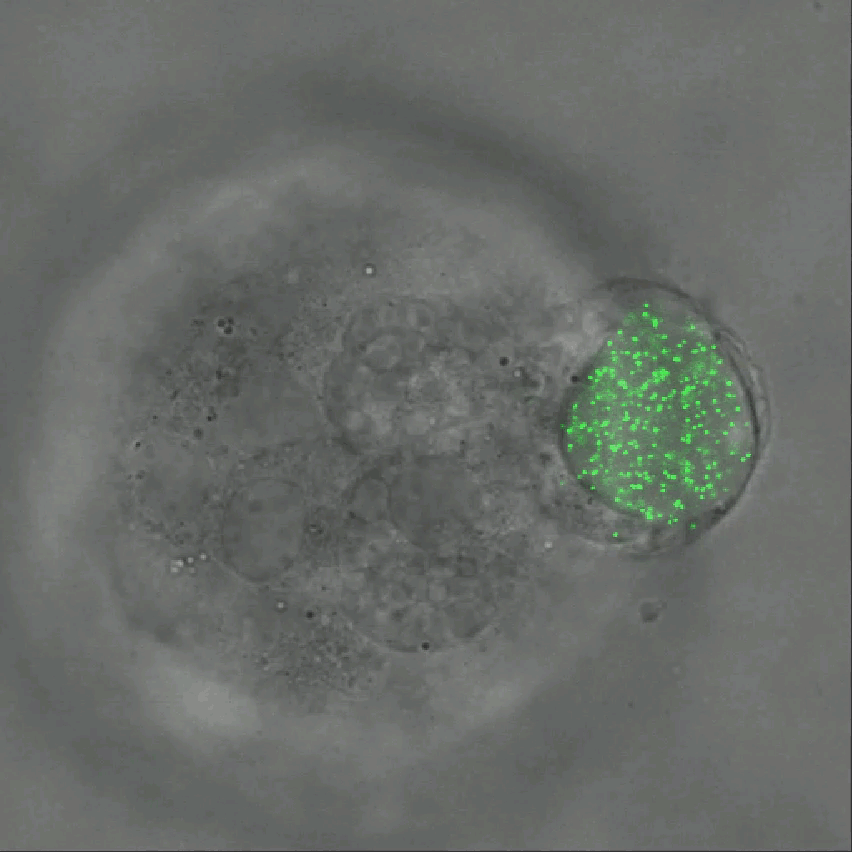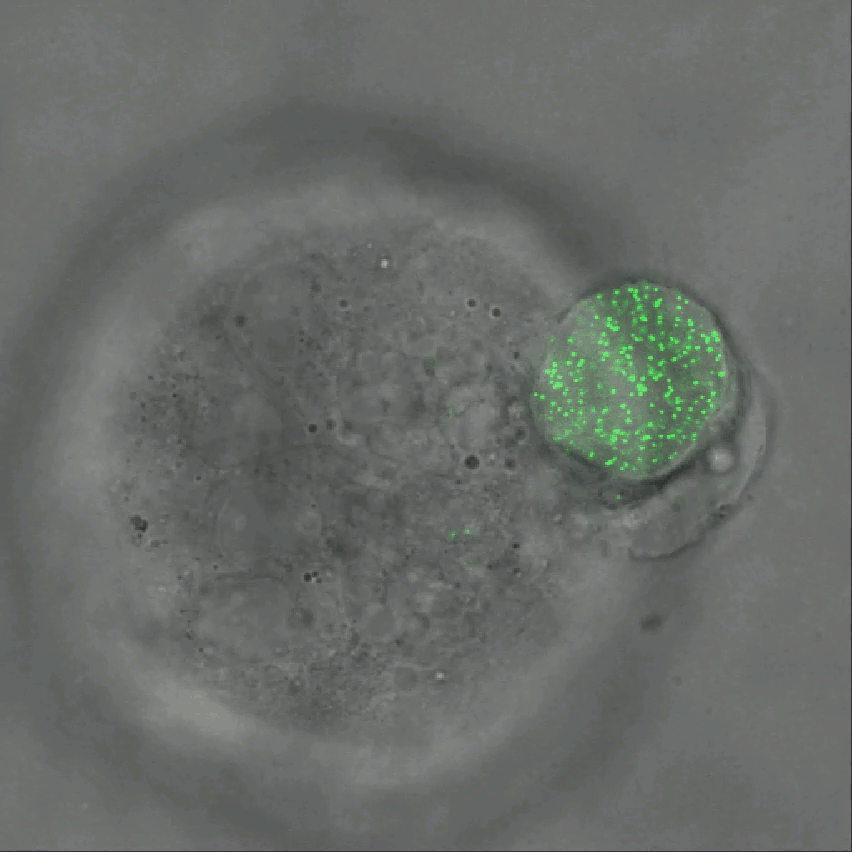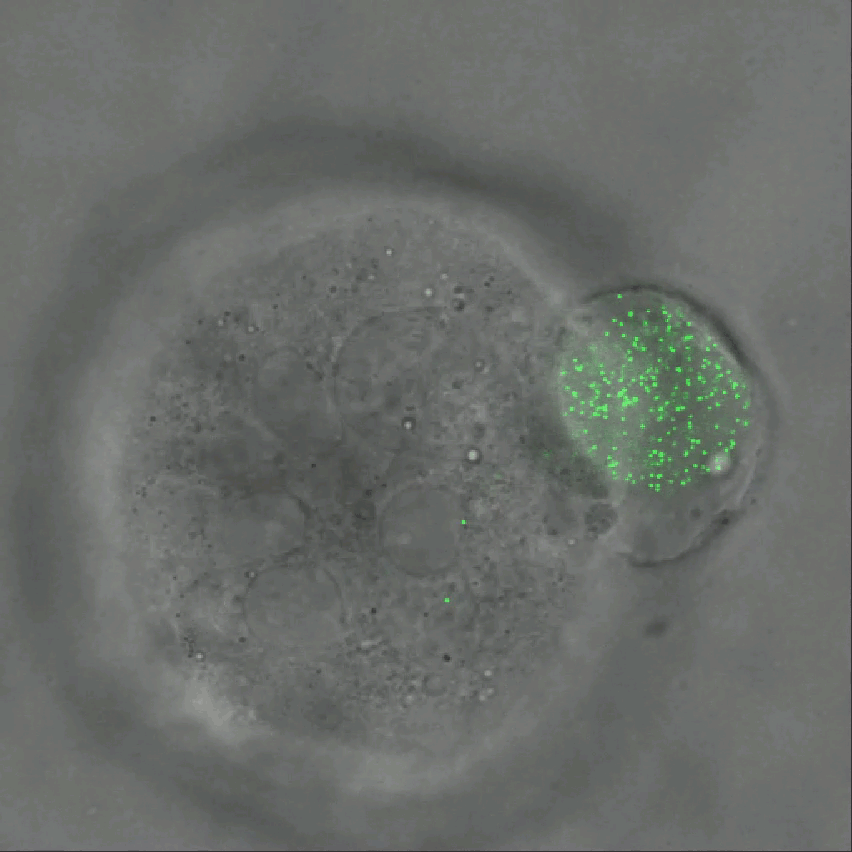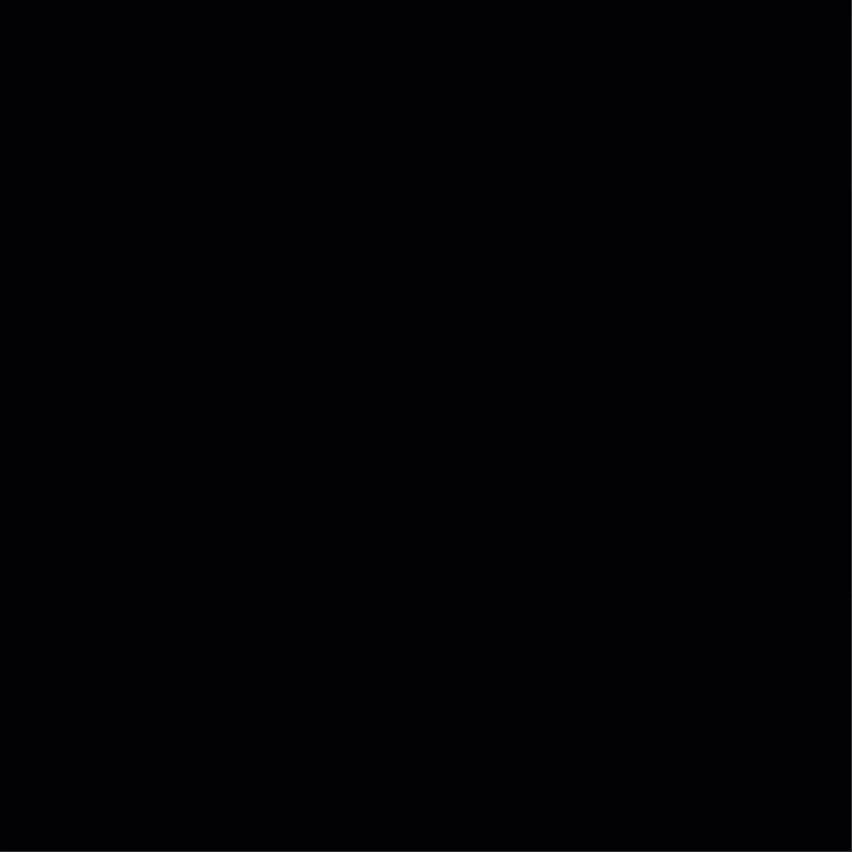
Credit: Ning Wang, University of Illinois at Urbana-Champaign
As tumor cells divide and grow, they push, pull, and squeeze one another. While scientists have suspected those mechanical stresses may play important roles in cancer, it’s been tough to figure out how. That’s in large part because there hadn’t been a good way to measure those forces within a tissue. Now, there is.
As described in Nature Communications, an NIH-funded research team has developed a technique for measuring those subtle mechanical forces in cancer and also during development [1]. Their ingenious approach is called the elastic round microgel (ERMG) method. It relies on round elastic microspheres—similar to miniature basketballs, only filled with fluorescent nanoparticles in place of air. In the time-lapse video above, you see growing and dividing melanoma cancer cells as they squeeze and spin one of those cell-sized “balls” over the course of 24 hours.
Creative Minds: Reprogramming the Brain
Posted on by Dr. Francis Collins

Caption: Neuronal circuits in the mouse retina. Cone photoreceptors (red) enable color vision; bipolar neurons (magenta) relay information further along the circuit; and a subtype of bipolar neuron (green) helps process signals sensed by other photoreceptors in dim light.
Credit: Brian Liu and Melanie Samuel, Baylor College of Medicine, Houston.
When most people think of reprogramming something, they probably think of writing code for a computer or typing commands into their smartphone. Melanie Samuel thinks of brain circuits, the networks of interconnected neurons that allow different parts of the brain to work together in processing information.
Samuel, a researcher at Baylor College of Medicine, Houston, wants to learn to reprogram the connections, or synapses, of brain circuits that function less well in aging and disease and limit our memory and ability to learn. She has received a 2016 NIH Director’s New Innovator Award to decipher the molecular cues that encourage the repair of damaged synapses or enable neurons to form new connections with other neurons. Because extensive synapse loss is central to most degenerative brain diseases, Samuel’s reprogramming efforts could help point the way to preventing or correcting wiring defects before they advance to serious and potentially irreversible cognitive problems.
Tumor Scanner Promises Fast 3D Imaging of Biopsies
Posted on by Dr. Francis Collins

Caption: University of Washington team that developed new light-sheet microscope (center) includes (l-r) Jonathan Liu, Adam Glaser, Larry True, Nicholas Reder, and Ye Chen.
Credit: Mark Stone/University of Washington
After surgically removing a tumor from a cancer patient, doctors like to send off some of the tissue for evaluation by a pathologist to get a better idea of whether the margins are cancer free and to guide further treatment decisions. But for technical reasons, completing the pathology report can take days, much to the frustration of patients and their families. Sometimes the results even require an additional surgical procedure.
Now, NIH-funded researchers have developed a groundbreaking new microscope to help perform the pathology in minutes, not days. How’s that possible? The device works like a scanner for tissues, using a thin sheet of light to capture a series of thin cross sections within a tumor specimen without having to section it with a knife, as is done with conventional pathology. The rapidly acquired 2D “optical sections” are processed by a computer that assembles them into a high-resolution 3D image for immediate analysis.
Got It Down Cold: Cryo-Electron Microscopy Named Method of the Year
Posted on by Dr. Francis Collins

Credit: Veronica Falconieri, Subramaniam Lab, National Cancer Institute
In the quest to find faster, better ways of mapping the structure of proteins and other key biological molecules, a growing number of researchers are turning to an innovative method that pushes the idea of a freeze frame to a whole new level: cryo-electron microscopy (cryo-EM). The technique, which involves flash-freezing molecules in liquid nitrogen and bombarding them with electrons to capture their images with a special camera, has advanced dramatically since its inception thanks to the efforts of many creative minds. In fact, cryo-EM has improved so much that its mapping performance now rivals that of X-ray crystallography [1], the long-time workhorse of drug developers and structural biologists.
To get an idea of just how far cryo-EM has come over the last decade, take a look at the composite image above, which shows a bacterial enzyme (beta-galactosidase) bound to a drug-like molecule (phenylethyl beta-D-thiogalactopyranoside). To the left, you see a blob-like area generated by cryo-EM methods that would have been considered state-of-the-art just a few years ago. To the right, there’s an exquisitely detailed structure, which was produced at more than 10-times greater resolution using the latest advances in cryo-EM. In fact, today’s cryo-EM is so powerful that researchers can almost make out individual atoms! Very impressive, and just one of the many reasons why the journal Nature Methods recently named cryo-EM its “Method of the Year” for 2015 [2].
Snapshots of Life: Allergen Art
Posted on by Dr. Francis Collins
Seasonal allergy sufferers, allow me to take advantage of the powers of confocal microscopy to introduce you to your tormenter: pollen. Although pollen grains look amazing at this magnification, their effects on many of us are not so wonderful. These spiky spheres can trigger an immune reaction that produces the runny nose, itchy eyes, and other symptoms that make people with pollen allergies miserable from early spring through late fall.
In fact, this image was created by a seasonal allergy sufferer who also happens to be a cell biologist. On a Saturday afternoon about a decade ago, Edna Cukierman of Philadelphia’s Fox Chase Cancer Center had just received a new spinning disc confocal microscope and couldn’t wait to try it out. There was just one catch—she also had to watch her two children. Not to be deterred, this multi-tasking scientist/mom turned the process of calibrating her new microscope into a creative way of entertaining her kids.