adult stem cells
Mini-Lungs in a Lab Dish Mimic Early COVID-19 Infection
Posted on by Dr. Francis Collins

Researchers have become skilled at growing an array of miniature human organs in the lab. Such lab-grown “organoids” have been put to work to better understand diabetes, fatty liver disease, color vision, and much more. Now, NIH-funded researchers have applied this remarkable lab tool to produce mini-lungs to study SARS-CoV-2, the coronavirus that causes COVID-19.
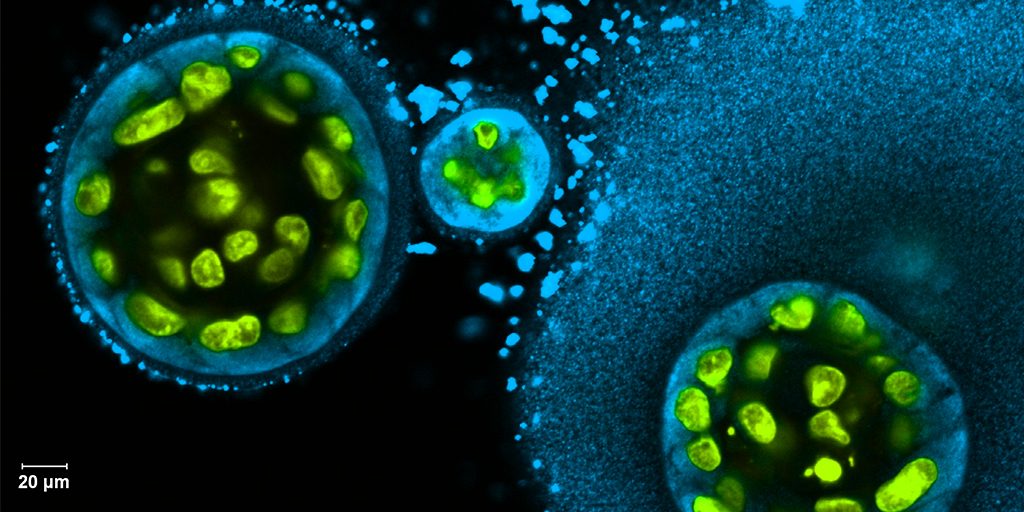
The intriguing bubble-like structures (red/clear) in the mini-lung pictured above represent developing alveoli, the tiny air sacs in our lungs, where COVID-19 infections often begin. In this organoid, the air sacs consist of many thousands of cells, all of which arose from a single adult stem cell isolated from tissues found deep within healthy human lungs. When carefully nurtured in lab dishes, those so-called alveolar epithelial type-2 cells (AT2s) begin to multiply. As they grow, they spontaneously assemble into structures that closely resemble alveoli.
A team led by Purushothama Rao Tata, Duke University School of Medicine, Durham, NC, developed these mini-lungs in a quest to understand how adult stem cells help to regenerate damaged tissue in the deepest recesses of the lungs, where SARS-CoV-2 attacks. In earlier studies, the researchers had shown it was possible for these cells to produce miniature alveoli. But there was a problem: the “soup” they used to nurture the growing cells included ingredients that weren’t well defined, making it hard to characterize the experiments fully.
In the study, now reported in Cell Stem Cell, the researchers found a way to simplify and define that brew. For the first time, they could produce mini-lungs consisting only of human lung cells. By growing them in large numbers in the lab, they can now learn more about SARS-CoV-2 infection and look for new ways to prevent or treat it.
Tata and his collaborators at the University of North Carolina, Chapel Hill, have already confirmed that SARS-CoV-2 infects the mini-lungs via the critical ACE2 receptor, just as the virus is known to do in the lungs of an infected person.
Interestingly, the cells also produce cytokines, inflammatory molecules that have been tied to tissue damage. The findings suggest the cytokine signals may come from the lungs themselves, even before immune cells arrive on the scene.
The heavily infected lung cells eventually self-destruct and die. In an unexpected turn of events, they even induce cell death in some neighboring healthy cells that are not infected. The relevance of the studies to the clinic was boosted by the finding that the gene activity patterns in the mini-lungs are a close match to those found in samples taken from six patients with severe COVID-19.
Now that he’s got the recipe down, Tata is busy making organoids and helping to model COVID-19 infections, with the hope of identifying and testing promising new treatments. It’s clear these mini-lungs are breathing some added life into the basic study of COVID-19.
Reference:
[1] Human lung stem cell-based alveolospheres provide insights into SARS-CoV-2-mediated interferon responses and pneumocyte dysfunction. Katsura H, Sontake V, Tata A, Kobayashi Y, Edwards CE, Heaton BE, Konkimalla A, Asakura T, Mikami Y, Fritch EJ, Lee PJ, Heaton NS, Boucher RC, Randell SH, Baric RS, Tata PR. Cell Stem Cell. 2020 Oct 21:S1934-5909(20)30499-9.
Links:
Coronavirus (COVID-19) (NIH)
Tata Lab (Duke University School of Medicine, Durham, NC)
NIH Support: National Institute of Allergy and Infectious Diseases; National Heart, Lung, and Blood Institute; National Institute of General Medical Sciences; National Institute of Diabetes and Digestive and Kidney Diseases
Studying Color Vision in a Dish
Posted on by Dr. Francis Collins

Credit: Eldred et al., Science
Researchers can now grow miniature versions of the human retina—the light-sensitive tissue at the back of the eye—right in a lab dish. While most “retina-in-a-dish” research is focused on finding cures for potentially blinding diseases, these organoids are also providing new insights into color vision.
Our ability to view the world in all of its rich and varied colors starts with the retina’s light-absorbing cone cells. In this image of a retinal organoid, you see cone cells (blue and green). Those labelled with blue produce a visual pigment that allows us to see the color blue, while those labelled green make visual pigments that let us see green or red. The cells that are labeled with red show the highly sensitive rod cells, which aren’t involved in color vision, but are very important for detecting motion and seeing at night.
The Science of Saliva
Posted on by Dr. Francis Collins
Credit: Swati Pradhan-Bhatt, Christiana Care Health System, Newark, DE
Whether it’s salmon sizzling on the grill or pizza fresh from the oven, you probably have a favorite food that makes your mouth water. But what if your mouth couldn’t water—couldn’t make enough saliva? When salivary glands stop working and the mouth becomes dry, either from disease or as a side effect of medical treatment, the once-routine act of eating can become a major challenge.
To help such people, researchers are now trying to engineer replacement salivary glands. While the research is still in the early stages, this image captures a crucial first step in the process: generating 3D structures of saliva-secreting cells (yellow). When grown on a scaffold of biocompatible polymers infused with factors to encourage development, these cells cluster into spherical structures similar to those seen in salivary glands. And they don’t just look like salivary cells, they act like them, producing the distinctive enzyme in saliva, alpha amylase (blue).
Helping People in Need of a Stem Cell Transplant
Posted on by Dr. Francis Collins

Caption: Study co-authors Jonathan Hoggatt (r) and Bin-Kuan Chou (l) look through a microscope at a patient’s mobilized stem cells.
Credit: Lee Hopkins, OLP Creative
In certain people with cancer or other serious diseases, transplants of healthy adult stem cells can be lifesaving. But donating blood-forming stem cells is a bit more complicated than giving blood. For example, stem-cell donors most often undergo five days of injections to build up enough of those vital cells in the blood for donation.
Wouldn’t it be great if we could find a way to make the donation process easier? Such improvements are now on the horizon.NIH-funded researchers recently found that, at least in mice, a single injection of two complementary treatments can generate enough stem cells in 15 minutes [1]. What’s more, stem cells harvested in this way have qualities that appear to increase the odds of transplant success.
Regenerative Medicine: Making Blood Stem Cells in the Lab
Posted on by Dr. Francis Collins

Caption: Arrow in first panel points to an endothelial cell induced to become hematopoietic stem cell (HSC). Second and third panels show the expansion of HSCs over time.
Credit: Raphael Lis, Weill Cornell Medicine, New York, NY
Bone marrow transplants offer a way to cure leukemia, sickle cell disease, and a variety of other life-threatening blood disorders.There are two major problems, however: One is many patients don’t have a well-matched donor to provide the marrow needed to reconstitute their blood with healthy cells. Another is even with a well-matched donor, rejection or graft versus host disease can occur, and lifelong immunosuppression may be needed.
A much more powerful option would be to develop a means for every patient to serve as their own bone marrow donor. To address this challenge, researchers have been trying to develop reliable, lab-based methods for making the vital, blood-producing component of bone marrow: hematopoietic stem cells (HSCs).
Two new studies by NIH-funded research teams bring us closer to achieving this feat. In the first study, researchers developed a biochemical “recipe” to produce HSC-like cells from human induced pluripotent stem cells (iPSCs), which were derived from mature skin cells. In the second, researchers employed another approach to convert mature mouse endothelial cells, which line the inside of blood vessels, directly into self-renewing HSCs. When these HSCs were transplanted into mice, they fully reconstituted the animals’ blood systems with healthy red and white blood cells.
Find and Replace: DNA Editing Tool Shows Gene Therapy Promise
Posted on by Dr. Francis Collins

Caption: This image represents an infection-fighting cell called a neutrophil. In this artist’s rendering, the cell’s DNA is being “edited” to help restore its ability to fight bacterial invaders.
Credit: NIAID, NIH
For gene therapy research, the perennial challenge has been devising a reliable way to insert safely a working copy of a gene into relevant cells that can take over for a faulty one. But with the recent discovery of powerful gene editing tools, the landscape of opportunity is starting to change. Instead of threading the needle through the cell membrane with a bulky gene, researchers are starting to design ways to apply these tools in the nucleus—to edit out the disease-causing error in a gene and allow it to work correctly.
While the research is just getting under way, progress is already being made for a rare inherited immunodeficiency called chronic granulomatous disease (CGD). As published recently in Science Translational Medicine, a team of NIH researchers has shown with the help of the latest CRISPR/Cas9 gene-editing tools, they can correct a mutation in human blood-forming adult stem cells that triggers a common form of CGD. What’s more, they can do it without introducing any new and potentially disease-causing errors to the surrounding DNA sequence [1].
When those edited human cells were transplanted into mice, the cells correctly took up residence in the bone marrow and began producing fully functional white blood cells. The corrected cells persisted in the animal’s bone marrow and bloodstream for up to five months, providing proof of principle that this lifelong genetic condition and others like it could one day be cured without the risks and limitations of our current treatments.

