biophysics
The Perfect Cytoskeletal Storm
Posted on by Dr. Francis Collins

Ever thought about giving cell biology a whirl? If so, I suggest you sit down and take a look at this full-blown cytoskeletal “storm,” which provides a spectacular dynamic view of the choreography of life.
Before a cell divides, it undergoes a process called mitosis that copies its chromosomes and produces two identical nuclei. As part of this process, microtubules, which are structural proteins that help make up the cell’s cytoskeleton, reorganize the newly copied chromosomes into a dense, football-shaped spindle. The position of this mitotic spindle tells the cell where to divide, allowing each daughter cell to contain its own identical set of DNA.
To gain a more detailed view of microtubules in action, researchers designed an experimental system that utilizes an extract of cells from the African clawed frog (Xenopus laevis). As the video begins, a star-like array of microtubules (red) radiate outward in an apparent effort to prepare for cell division. In this configuration, the microtubules continually adjust their lengths with the help of the protein EB-1 (green) at their tips. As the microtubules grow and bump into the walls of a lab-generated, jelly-textured enclosure (dark outline), they buckle—and the whole array then whirls around the center.
Abdullah Bashar Sami, a Ph.D. student in the NIH-supported lab of Jesse “Jay” Gatlin, University of Wyoming, Laramie, shot this movie as a part his basic research to explore the still poorly understood physical forces generated by microtubules. The movie won first place in the 2019 Green Fluorescent Protein Image and Video Contest sponsored by the American Society for Cell Biology. The contest honors the 25th anniversary of the discovery of green fluorescent protein (GFP), which transformed cell biology and earned the 2008 Nobel Prize in Chemistry for three scientists who had been supported by NIH.
Like many movies, the setting was key to this video’s success. The video was shot inside a microfluidic chamber, designed in the Gatlin lab, to study the physics of microtubule assembly just before cells divide. The tiny chamber holds a liquid droplet filled with the cell extract.
When the liquid is exposed to an ultra-thin beam of light, it forms a jelly-textured wall, which traps the molecular contents inside [1]. Then, using time-lapse microscopy, the researchers watch the mechanical behavior of GFP-labeled microtubules [2] to see how they work to position the mitotic spindle. To do this, microtubules act like shapeshifters—scaling to adjust to differences in cell size and geometry.
The Gatlin lab is continuing to use their X. laevis system to ask fundamental questions about microtubule assembly. For many decades, both GFP and this amphibian model have provided cell biologists with important insights into the choreography of life, and, as this work shows, we can expect much more to come!
References:
[1] Microtubule growth rates are sensitive to global and local changes in microtubule plus-end density. Geisterfer ZM, Zhu D, Mitchison T, Oakey J, Gatlin JC. November 20, 2019.
[2] Tau-based fluorescent protein fusions to visualize microtubules. Mooney P, Sulerud T, Pelletier JF, Dilsaver MR, et al. Cytoskeleton (Hoboken). 2017 Jun;74(6):221-232.
Links:
Mitosis (National Human Genome Research Institute/NIH)
Gatlin Lab (University of Wyoming, Laramie)
Green Fluorescent Protein Image and Video Contest (American Society for Cell Biology, Bethesda, MD)
2008 Nobel Prize in Chemistry (Nobel Foundation, Stockholm, Sweden)
NIH Support: National Institute of General Medical Sciences
Watching Cancer Cells Play Ball
Posted on by Dr. Francis Collins
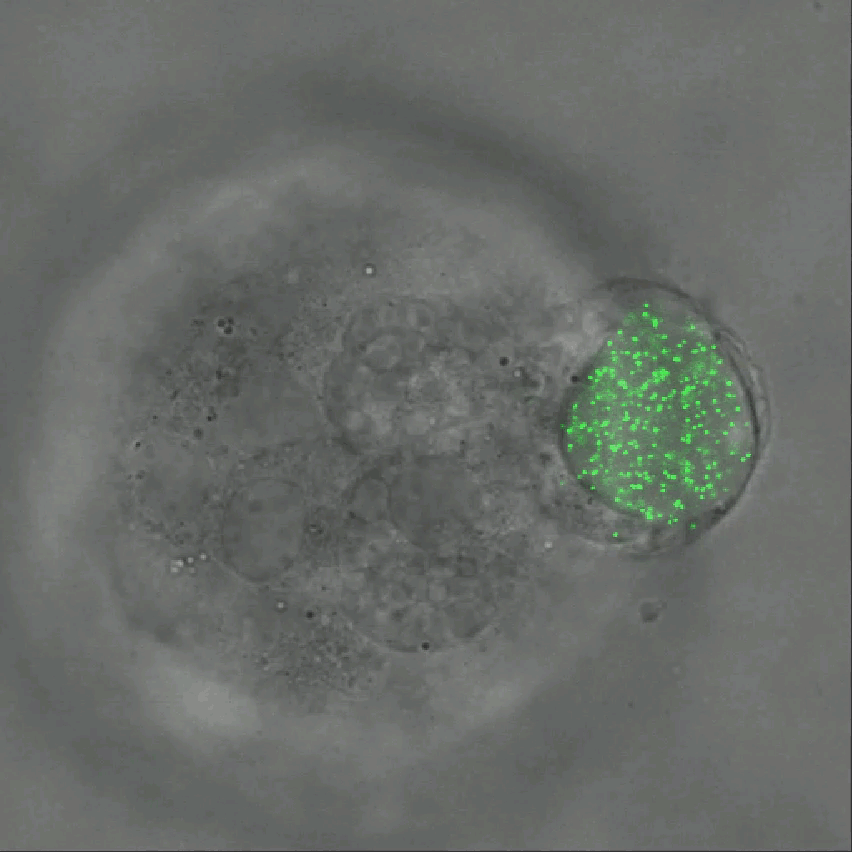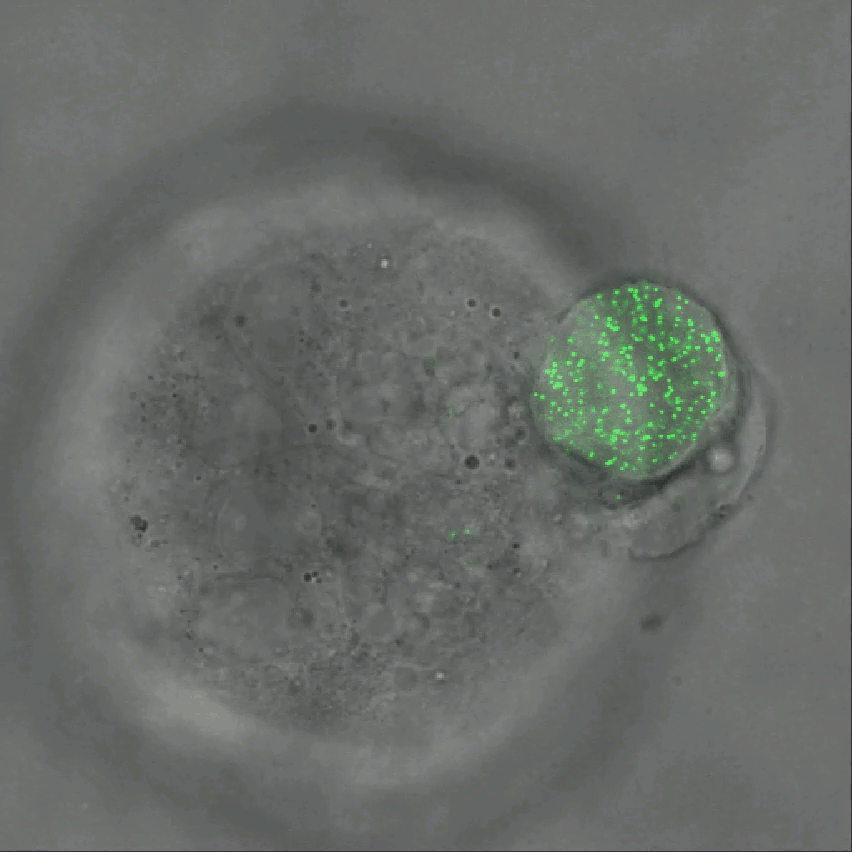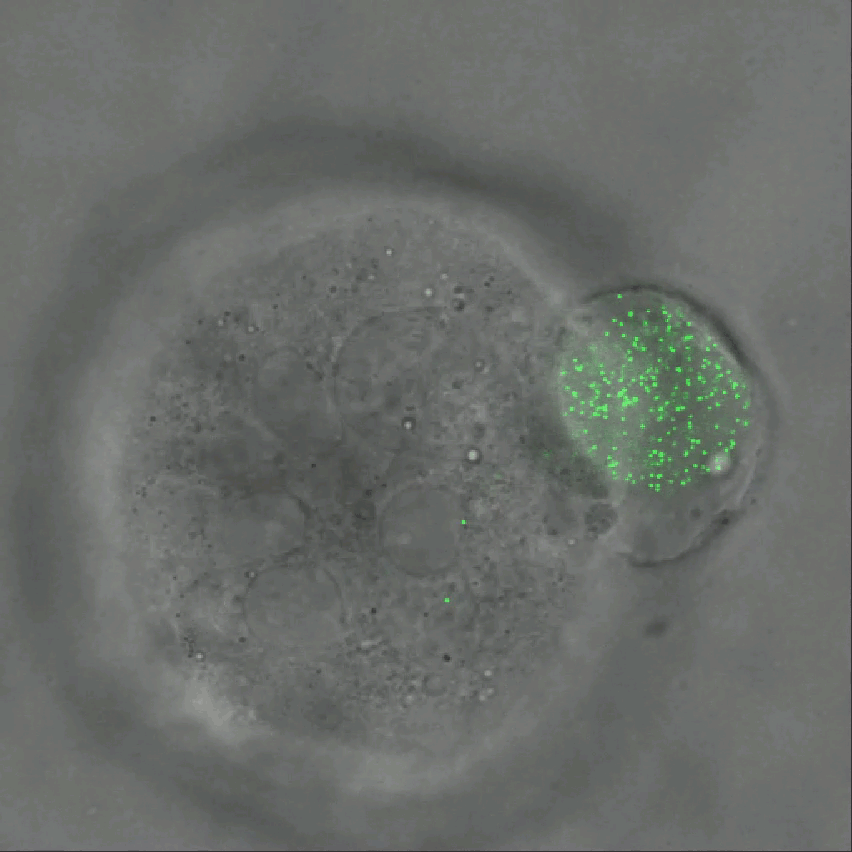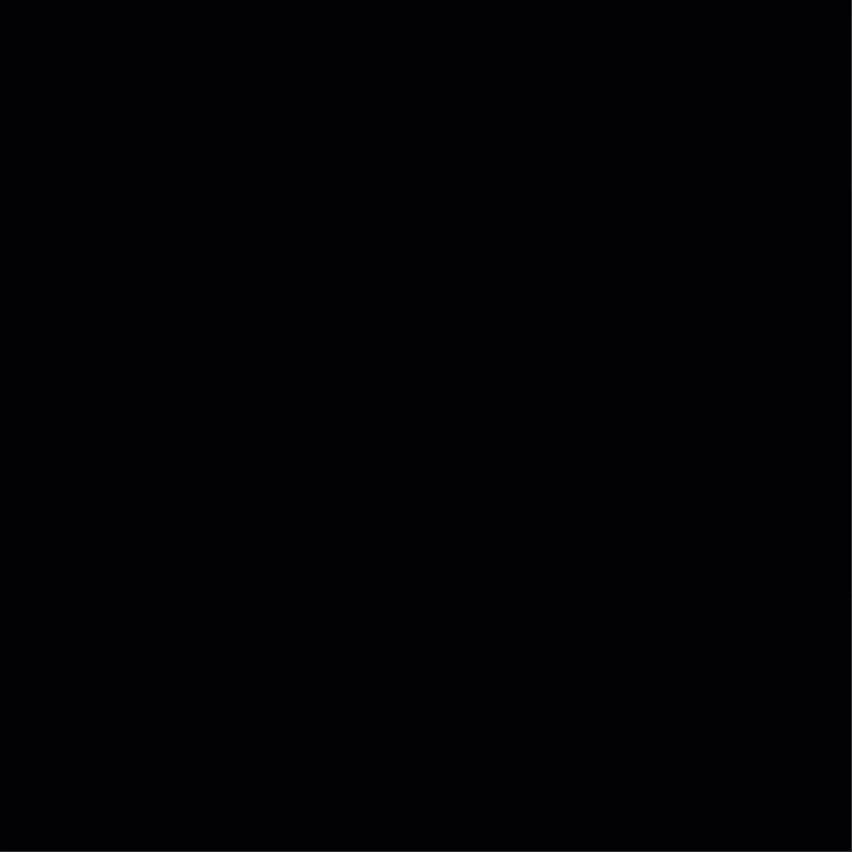
Credit: Ning Wang, University of Illinois at Urbana-Champaign
As tumor cells divide and grow, they push, pull, and squeeze one another. While scientists have suspected those mechanical stresses may play important roles in cancer, it’s been tough to figure out how. That’s in large part because there hadn’t been a good way to measure those forces within a tissue. Now, there is.
As described in Nature Communications, an NIH-funded research team has developed a technique for measuring those subtle mechanical forces in cancer and also during development [1]. Their ingenious approach is called the elastic round microgel (ERMG) method. It relies on round elastic microspheres—similar to miniature basketballs, only filled with fluorescent nanoparticles in place of air. In the time-lapse video above, you see growing and dividing melanoma cancer cells as they squeeze and spin one of those cell-sized “balls” over the course of 24 hours.
Snapshots of Life: Biological Bubble Machine
Posted on by Dr. Francis Collins
As kids, most of us got a bang out of blowing soap bubbles and watching them float around. Biologists have learned that some of our cells do that too. On the right, you can see two cells (greenish yellow) in the process of forming bubbles, or plasma membrane vesicles (PMVs). During this blebbing process, a cell’s membrane temporarily disassociates from its underlying cytoskeleton, forming a tiny pouch that, over the course of about 30 minutes, is “inflated” with a mix of proteins and lipids from inside the cell. After the PMVs are fully filled, these bubble-like structures are pinched off and released, like those that you see in the background. Certain cells constantly release PMVs, along with other types of vesicles, and may use those to communicate with other cells throughout the body.
This particular image, an entrant in the Biophysical Society’s 2017 Art of Science Image Contest, was produced by researchers working in the NIH-supported lab of Jeanne Stachowiak at the University of Texas at Austin. Stachowiak’s group is among the first to explore the potential of PMVs as specialized drug-delivery systems to target cancer and other disorders [1].
Until recently, most efforts to exploit vesicles for therapeutic uses have employed synthetic versions of a different type of vesicle, called an exosome. But Stachowiak and others have realized that PMVs come with certain built-in advantages. A major one is that a patient’s own cells could in theory serve as the production facility.
Snapshots of Life: Imperfect but Beautiful Intruder
Posted on by Dr. Francis Collins

Credit: Boon Chong Goh, Beckman Institute, University of Illinois at Urbana-Champaign
The striking image you see above is an example of what can happen when scientists combine something old with something new. In this case, a researcher took the Rous sarcoma virus (RSV)—a virus that’s been studied for more than century because of its ability to cause cancer in chickens and the insights it provided on human oncogenes [1, 2]—and used modern computational tools to generate a model of its atomic structure.
Here you see an immature RSV particle that’s just budded from an infected chicken cell and entered the avian bloodstream. A lattice of proteins (red) held together by short peptides (green) cover the outer shell of the immature virus, shielding other proteins (blue) that make up an inner shell.

