cytoskeleton
The Perfect Cytoskeletal Storm
Posted on by Dr. Francis Collins

Ever thought about giving cell biology a whirl? If so, I suggest you sit down and take a look at this full-blown cytoskeletal “storm,” which provides a spectacular dynamic view of the choreography of life.
Before a cell divides, it undergoes a process called mitosis that copies its chromosomes and produces two identical nuclei. As part of this process, microtubules, which are structural proteins that help make up the cell’s cytoskeleton, reorganize the newly copied chromosomes into a dense, football-shaped spindle. The position of this mitotic spindle tells the cell where to divide, allowing each daughter cell to contain its own identical set of DNA.
To gain a more detailed view of microtubules in action, researchers designed an experimental system that utilizes an extract of cells from the African clawed frog (Xenopus laevis). As the video begins, a star-like array of microtubules (red) radiate outward in an apparent effort to prepare for cell division. In this configuration, the microtubules continually adjust their lengths with the help of the protein EB-1 (green) at their tips. As the microtubules grow and bump into the walls of a lab-generated, jelly-textured enclosure (dark outline), they buckle—and the whole array then whirls around the center.
Abdullah Bashar Sami, a Ph.D. student in the NIH-supported lab of Jesse “Jay” Gatlin, University of Wyoming, Laramie, shot this movie as a part his basic research to explore the still poorly understood physical forces generated by microtubules. The movie won first place in the 2019 Green Fluorescent Protein Image and Video Contest sponsored by the American Society for Cell Biology. The contest honors the 25th anniversary of the discovery of green fluorescent protein (GFP), which transformed cell biology and earned the 2008 Nobel Prize in Chemistry for three scientists who had been supported by NIH.
Like many movies, the setting was key to this video’s success. The video was shot inside a microfluidic chamber, designed in the Gatlin lab, to study the physics of microtubule assembly just before cells divide. The tiny chamber holds a liquid droplet filled with the cell extract.
When the liquid is exposed to an ultra-thin beam of light, it forms a jelly-textured wall, which traps the molecular contents inside [1]. Then, using time-lapse microscopy, the researchers watch the mechanical behavior of GFP-labeled microtubules [2] to see how they work to position the mitotic spindle. To do this, microtubules act like shapeshifters—scaling to adjust to differences in cell size and geometry.
The Gatlin lab is continuing to use their X. laevis system to ask fundamental questions about microtubule assembly. For many decades, both GFP and this amphibian model have provided cell biologists with important insights into the choreography of life, and, as this work shows, we can expect much more to come!
References:
[1] Microtubule growth rates are sensitive to global and local changes in microtubule plus-end density. Geisterfer ZM, Zhu D, Mitchison T, Oakey J, Gatlin JC. November 20, 2019.
[2] Tau-based fluorescent protein fusions to visualize microtubules. Mooney P, Sulerud T, Pelletier JF, Dilsaver MR, et al. Cytoskeleton (Hoboken). 2017 Jun;74(6):221-232.
Links:
Mitosis (National Human Genome Research Institute/NIH)
Gatlin Lab (University of Wyoming, Laramie)
Green Fluorescent Protein Image and Video Contest (American Society for Cell Biology, Bethesda, MD)
2008 Nobel Prize in Chemistry (Nobel Foundation, Stockholm, Sweden)
NIH Support: National Institute of General Medical Sciences
Seeing the Cytoskeleton in a Whole New Light
Posted on by Dr. Francis Collins

It’s been 25 years since researchers coaxed a bacterium to synthesize an unusual jellyfish protein that fluoresced bright green when irradiated with blue light. Within months, another group had also fused this small green fluorescent protein (GFP) to larger proteins to make their whereabouts inside the cell come to light—like never before.
To mark the anniversary of this Nobel Prize-winning work and show off the rainbow of color that is now being used to illuminate the inner workings of the cell, the American Society for Cell Biology (ASCB) recently held its Green Fluorescent Protein Image and Video Contest. Over the next few months, my blog will feature some of the most eye-catching entries—starting with this video that will remind those who grew up in the 1980s of those plasma balls that, when touched, light up with a simulated bolt of colorful lightning.
This video, which took third place in the ASCB contest, shows the cytoskeleton of a frequently studied human breast cancer cell line. The cytoskeleton is made from protein structures called microtubules, made visible by fluorescently tagging a protein called doublecortin (orange). Filaments of another protein called actin (purple) are seen here as the fine meshwork in the cell periphery.
The cytoskeleton plays an important role in giving cells shape and structure. But it also allows a cell to move and divide. Indeed, the motion in this video shows that the complex network of cytoskeletal components is constantly being organized and reorganized in ways that researchers are still working hard to understand.
Jeffrey van Haren, Erasmus University Medical Center, Rotterdam, the Netherlands, shot this video using the tools of fluorescence microscopy when he was a postdoctoral researcher in the NIH-funded lab of Torsten Wittman, University of California, San Francisco.
All good movies have unusual plot twists, and that’s truly the case here. Though the researchers are using a breast cancer cell line, their primary interest is in the doublecortin protein, which is normally found in association with microtubules in the developing brain. In fact, in people with mutations in the gene that encodes this protein, neurons fail to migrate properly during development. The resulting condition, called lissencephaly, leads to epilepsy, cognitive disability, and other neurological problems.
Cancer cells don’t usually express doublecortin. But, in some of their initial studies, the Wittman team thought it would be much easier to visualize and study doublecortin in the cancer cells. And so, the researchers tagged doublecortin with an orange fluorescent protein, engineered its expression in the breast cancer cells, and van Haren started taking pictures.
This movie and others helped lead to the intriguing discovery that doublecortin binds to microtubules in some places and not others [1]. It appears to do so based on the ability to recognize and bind to certain microtubule geometries. The researchers have since moved on to studies in cultured neurons.
This video is certainly a good example of the illuminating power of fluorescent proteins: enabling us to see cells and their cytoskeletons as incredibly dynamic, constantly moving entities. And, if you’d like to see much more where this came from, consider visiting van Haren’s Twitter gallery of microtubule videos here:
Reference:
[1] Doublecortin is excluded from growing microtubule ends and recognizes the GDP-microtubule lattice. Ettinger A, van Haren J, Ribeiro SA, Wittmann T. Curr Biol. 2016 Jun 20;26(12):1549-1555.
Links:
Lissencephaly Information Page (National Institute of Neurological Disorders and Stroke/NIH)
Wittman Lab (University of California, San Francisco)
Green Fluorescent Protein Image and Video Contest (American Society for Cell Biology, Bethesda, MD)
NIH Support: National Institute of General Medical Sciences
The Actin Superhighway
Posted on by Dr. Francis Collins

Credit: Andrew Lombardo and David Warshaw, University of Vermont, Burlington
What looks like a traffic grid filled with roundabouts is nothing of the sort: It’s actually a peek inside a tiny microchamber that models a complex system operating in many of our cells. The system is a molecular transportation network made of the protein actin, and researchers have reconstructed it in the lab to study its rules of the road and, when things go wrong, how it can lead to molecular traffic accidents.
This 3D super-resolution image shows the model’s silicone beads (circles) positioned in a tiny microfluidic-chamber. Suspended from the beads are actin filaments that form some of the main cytoskeletal roadways in our cells. Interestingly, a single dye creates the photo’s beautiful colors, which arise from the different vertical dimensions of a microscopic image: 300 nanometers below the focus (red), at focus (green), and 300 nanometers above the focus (blue). When a component spans multiple dimensions—such as the spherical beads—all the colors of the rainbow are visible. The technique is called 3D stochastic optical reconstruction microscopy, or STORM [1].
Snapshots of Life: Biological Bubble Machine
Posted on by Dr. Francis Collins
As kids, most of us got a bang out of blowing soap bubbles and watching them float around. Biologists have learned that some of our cells do that too. On the right, you can see two cells (greenish yellow) in the process of forming bubbles, or plasma membrane vesicles (PMVs). During this blebbing process, a cell’s membrane temporarily disassociates from its underlying cytoskeleton, forming a tiny pouch that, over the course of about 30 minutes, is “inflated” with a mix of proteins and lipids from inside the cell. After the PMVs are fully filled, these bubble-like structures are pinched off and released, like those that you see in the background. Certain cells constantly release PMVs, along with other types of vesicles, and may use those to communicate with other cells throughout the body.
This particular image, an entrant in the Biophysical Society’s 2017 Art of Science Image Contest, was produced by researchers working in the NIH-supported lab of Jeanne Stachowiak at the University of Texas at Austin. Stachowiak’s group is among the first to explore the potential of PMVs as specialized drug-delivery systems to target cancer and other disorders [1].
Until recently, most efforts to exploit vesicles for therapeutic uses have employed synthetic versions of a different type of vesicle, called an exosome. But Stachowiak and others have realized that PMVs come with certain built-in advantages. A major one is that a patient’s own cells could in theory serve as the production facility.
Cool Videos: Pushing the Limits of Live-Cell Microscopy
Posted on by Dr. Francis Collins
If you’re not watching recent work in biology, you might have thought that light microscopy hit its limits years ago. After all, it’s been around a long time. But to the contrary, microscopic imaging technology just keeps getting better and better. Here you can look with unprecedented clarity at just one of the many dynamic processes going on within a living cell. Specifically, this video shows actin fibers (orange-red), which are key components of the cell’s cytoskeleton, slowly pulling clathrin-coated pits (green), which are basket-like structures containing molecular cargo, away from the cell’s external membrane and deeper within the cell.
This remarkable live-action view was produced using one of two new forms of extended-resolution, structured illumination microscopy (SIM). SIM is faster than other forms of super-resolution fluorescence microscopy. It’s also less damaging to cells, making it the go-to method for live-cell imaging. The downside has been SIM’s limited resolution—just twice that of conventional light microscopes. However, Nobel Prize-winner Eric Betzig and postdoc Dong Li of Howard Hughes Medical Institute, Janelia Research Campus, Ashburn, VA, along with colleagues including Jordan Beach and John Hammer at NIH’s National Heart, Lung, and Blood Institute, recently came up with two different solutions to enhance SIM’s spatial resolution.
Snapshots of Life: Cell Skeleton on the Move
Posted on by Dr. Francis Collins
Cells are constantly on the move. They shift, grow, and migrate to new locations—for example, to heal a wound or to intercept an infectious agent as part of an immune response. But how do cells actually move?
In this image, Torsten Wittmann, an NIH-funded cell biologist at the University of California, San Francisco, reveals the usually-invisible cytoskeleton of a normal human skin cell that lends the cell its mobility. The cytoskeleton is made from protein structures called microtubules—the wispy threads surrounding the purple DNA-containing nucleus—and filaments of a protein called actin, seen here as the fine blue meshwork in the cell periphery. Both actin and microtubules are critical for growth and movement.
The Beauty of Smooth Muscle
Posted on by Dr. Francis Collins
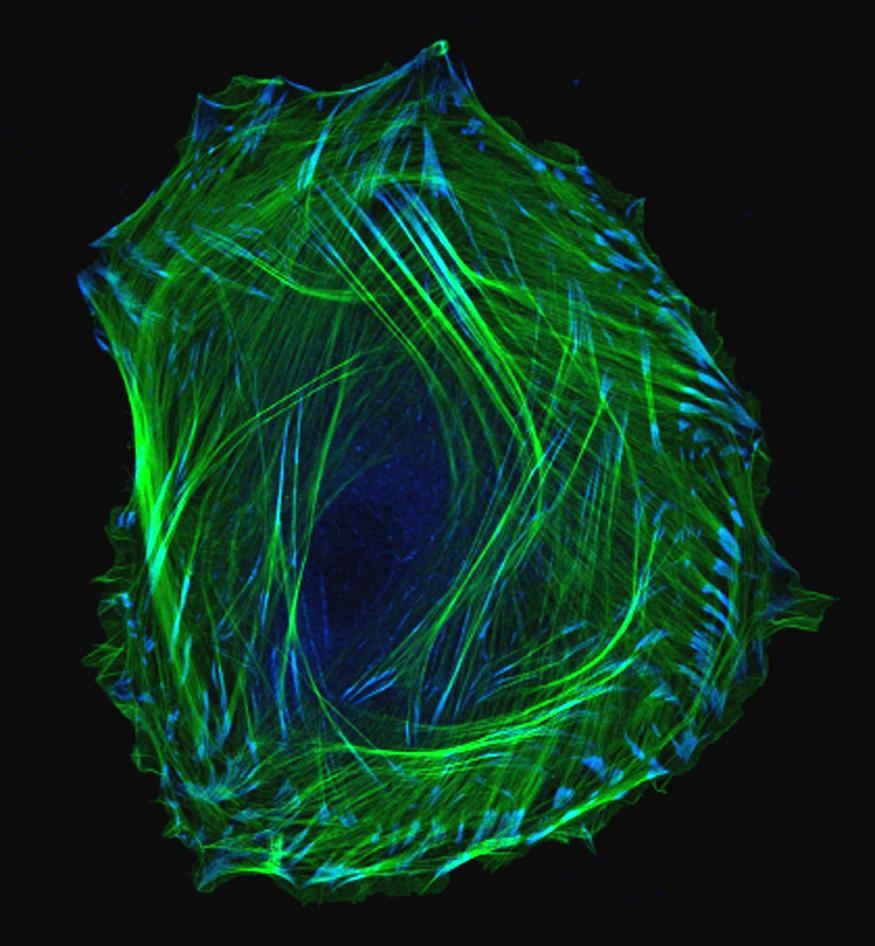
We humans have long wondered how, exactly, we develop from embryos into adults. This photo of an embryonic smooth muscle cell hints at the tremendous complexity of this fundamental biological mystery. And for those of you who might be wondering just what smooth muscles are, they’re the involuntary muscles found in places like the walls of our blood vessels, the digestive tract, the bladder, and the respiratory system.
This exquisite photo was produced using laser scanning confocal microscopy — a precise imaging method that includes the dimension of depth for scientific analysis. Here, green is used to label thin filaments of the protein actin, which is a key component of the cell’s cytoskeleton, and blue indicates another protein, called vinculin, which is enriched in locations involved in cell-cell adhesion.
Slowly but surely, using all the technology and tools available to us, we are unraveling the mysteries of biology — and turning our discoveries into health.



