iPS
Modeling Hypertrophic Cardiomyopathy in a Dish
Posted on by Dr. Francis Collins

Credit: Zhen Ma, University of California, Berkeley
Researchers have learned in recent years how to grow miniature human hearts in a dish. These “organoids” beat like the real thing and have allowed researchers to model many key aspects of how the heart works. What’s been really tough to model in a dish is how stresses on hearts that are genetically abnormal, such as in inherited familial cardiomyopathies, put people at greater risk for cardiac problems.
Enter the lab-grown human cardiac tissue pictured above. This healthy tissue comprised of the heart’s muscle cells, or cardiomyocytes (green, nuclei in red), was derived from induced pluripotent stem (iPS) cells. These cells are derived from adult skin or blood cells that are genetically reprogrammed to have the potential to develop into many different types of cells, including cardiomyocytes.
Zika and Birth Defects: The Evidence Mounts
Posted on by Dr. Francis Collins

Caption: Human neural progenitor cells (gray) infected with Zika virus (green) increased the enzyme caspase-3 (red), suggesting increased cell death.
Credit: Sarah C. Ogden, Florida State University, Tallahassee
Recently, public health officials have raised major concerns over the disturbing spread of the mosquito-borne Zika virus among people living in and traveling to many parts of Central and South America [1]. While the symptoms of Zika infection are typically mild, grave concerns have arisen about its potential impact during pregnancy. The concerns stem from the unusual number of births of children with microcephaly, a very serious condition characterized by a small head and damaged brain, coinciding with the spread of Zika virus. Now, two new studies strengthen the connection between Zika and an array of birth defects, including, but not limited to, microcephaly.
In the first study, NIH-funded laboratory researchers show that Zika virus can infect and kill human neural progenitor cells [2]. Those progenitor cells give rise to the cerebral cortex, a portion of the brain often affected in children with microcephaly. The second study, involving a small cohort of women diagnosed with Zika virus during their pregnancies in Rio de Janeiro, Brazil, suggests that the attack rate is disturbingly high, and microcephaly is just one of many risks to the developing fetus. [3]
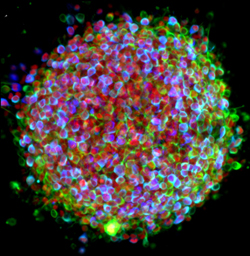
Why We’re So Excited About Stem Cells
Posted on by Dr. Francis Collins
Certainly – as you can see here – stem cells are spectacularly beautiful. But they also hold spectacular promise for medicine. That’s why I immediately expressed my enthusiasm for Monday’s Supreme Court ruling that effectively enables NIH to continue conducting and funding responsible, scientifically worthy stem cell research.
There are many kinds of stem cells. This is a picture of induced pluripotent stem cells – or, iPS cells. Investigators have recently begun using iPS cells to model several neurological diseases – including Parkinson’s. The cells here have been treated with growth factors that coax them into becoming the dopamine producing (dopaminergic) neurons lost in Parkinson’s. The colorized markers indicate the presence of three proteins found within dopaminergic neurons: (1) the enzyme needed to produce dopamine (tyrosine hydroxylase, in blue), (2) a structural protein specific to neurons (Type III beta-tubulin, in green), and (3) a gene regulatory protein needed in dopaminergic neurons (FOXA2, in red). The color-mixing in some cells indicates that all three proteins are present – confirming that these cells are on their way to becoming dopaminergic neurons.
Today’s image is more than just a pretty picture. It’s a window into the ways that disease affects the body – and possibly the ways we might counter those affects. The NIH/NINDS web site has more information about how iPS cells are being used to study Parkinson’s and other neurological disorders.