therapeutics
ACTIV Update: Making Major Strides in COVID-19 Therapeutic Development
Posted on by Dr. Francis Collins

Right now, many U.S. hospitals are stretched to the limit trying to help people battling serious cases of COVID-19. But as traumatic as this experience still is for patients and their loved ones, the chances of surviving COVID-19 have in fact significantly improved in the year since the start of the pandemic.
This improvement stems from several factors, including the FDA’s emergency use authorization (EUA) of a number of therapies found to be safe and effective for COVID-19. These include drugs that you may have heard about on the news: remdesivir (an antiviral), dexamethasone (a steroid), and monoclonal antibodies from the companies Eli Lilly and Regeneron.
Yet the quest to save more lives from COVID-19 isn’t even close to being finished, and researchers continue to work intensively to develop new and better treatments. A leader in this critical effort is NIH’s Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV) initiative, a public-private partnership involving 20 biopharmaceutical companies, academic experts, and multiple federal agencies.
ACTIV was founded last April to accelerate drug research that typically requires more than a decade of clinical ups and downs to develop a safe, effective therapy. And ACTIV has indeed moved at unprecedented speed since its launch. Cutting through the usual red tape and working with an intense sense of purpose, the partnership took a mere matter of weeks to set up its first four clinical trials. Beyond the agents mentioned above that have already been granted an EUA, ACTIV is testing 15 additional potential agents, with several of these already demonstrating promising results.
Here’s how ACTIV works. The program relies on four expert “working groups” with specific charges:
Preclinical Working Group: Shares standardized preclinical evaluation resources and accelerate testing of candidate therapies and vaccines for clinical trials.
Therapeutics Clinical Working Group: Prioritizes therapeutic agents for testing within an adaptive master protocol strategy for clinical research.
Clinical Trial Capacity Working Group: Has developed and organized an inventory of clinical trial capacity that can serve as potential settings in which to implement effective COVID-19 clinical trials.
Vaccines Working Group: Accelerates the evaluation of vaccine candidates.
To give you just one example of how much these expert bodies have accomplished in record time, the Therapeutics Clinical Working Group got to work immediately evaluating some 400 candidate therapeutics using multiple publicly available information sources. These candidates included antivirals, host-targeted immune modulators, monoclonal antibodies (mAb), and symptomatic/supportive agents including anticoagulants. To follow up on even more new leads, the working group launched a COVID-19 Clinical & Preclinical Candidate Compound Portal, which remains open for submissions of therapeutic ideas and data.
All the candidate agents have been prioritized using rigorous scoring and assessment criteria. What’s more, the working group simultaneously developed master protocols appropriate for each of the drug classes selected and patient populations: outpatient, inpatient, or convalescent.
Through the coordinated efforts of all the working groups, here’s where we stand with the ACTIV trials:
ACTIV-1: A large-scale Phase 3 trial is enrolling hospitalized adults to test the safety and effectiveness of three medicines (cenicriviroc, abatacept, and infliximab). They are called immune modulators because they help to minimize the effects of an overactive immune response in some COVID-19 patients. This response, called a “cytokine storm,” can lead to acute respiratory distress syndrome, multiple organ failure, and other life-threatening complications.
ACTIV-2: A Phase 2/3 trial is enrolling adults with COVID-19 who are not hospitalized to evaluate the safety of multiple monoclonal antibodies (Lilly’s LY-CoV555, Brii Biosciences’s BRII-196 and BRII-198, and AstraZeneca’s AZD7442) used to block or neutralize the SARS-CoV-2 virus. The Lilly monoclonal antibody LY-CoV555 received an EUA for high risk non-hospitalized patients on November 9, 2020 and ACTIV-2 continued to test the agent in an open label study to further determine safety and efficacy in outpatients. Another arm of this trial has just started, testing inhaled, easy-to-administer interferon beta-1a treatment in adults with mild-to-moderate COVID-19 who are not hospitalized. An additional arm will test the drug camostat mesilate, a protease inhibitor that can block the TMPRSS2 host protein that is necessary for viral entry into human cells.
ACTIV-3: This Phase 3 trial is enrolling hospitalized adults with COVID-19. This study primarily aims to evaluate safety and to understand if monoclonal antibodies (AstraZeneca’s AZD7442, BRII-196 and BRII-198, and the VIR-7831 from GSK/Vir Biotechnology) and potentially other types of therapeutics can reduce time to recovery. It also aims to understand a treatment’s effect on extrapulmonary complications and respiratory dysfunction. Lilly’s monoclonal antibody LY-CoV555 was one of the first agents to be tested in this clinical trial and it was determined to not show the same benefits seen in outpatients. [Update: NIH-Sponsored ACTIV-3 Clinical Trial Closes Enrollment into Two Sub-Studies, March 4, 2021]
ACTIV-4: This trial aims to determine if various types of blood thinners, including apixaban, aspirin, and both unfractionated (UF) and low molecular weight (LMW) heparin, can treat adults diagnosed with COVID-19 and prevent life-threatening blood clots from forming. There are actually three Phase 3 trials included in ACTIV-4. One is enrolling people diagnosed with COVID-19 but who are not hospitalized; a second is enrolling patients who are hospitalized; and a third is enrolling people who are recovering from COVID-19. ACTIV-4 has already shown that full doses of heparin blood thinners are safe and effective for moderately ill hospitalized patients.
ACTIV-5: This is a Phase 2 trial testing newly identified agents that might have a major benefit to hospitalized patients with COVID-19, but that need further “proof of concept” testing before they move into a registrational Phase 3 trial. (In fact, another name for this trial is the “Big Effect Trial”.) It is testing medicines previously developed for other conditions that might be beneficial in treatment of COVID-19. The first two agents being tested are risankizumab (the result of a collaboration between Boehringer-Ingelheim), which is already FDA-approved to treat plaque psoriasis, and lenzilumab, which is under development by Humanigen to treat patients experiencing cytokine storm as part of cancer therapy.
In addition to trials conducted under the ACTIV partnership, NIH has prioritized and tested additional therapeutics in “ACTIV-associated trials.” These are NIH-funded, randomized, placebo-controlled clinical trials with one or more industry partners. Here’s a table with a comprehensive list.
Looking a bit further down the road, we also seek to develop orally administered drugs that would potentially block the replication ability of SARS-CoV-2, the coronavirus that causes COVID-19, in the earliest stages of infection. One goal would be to develop an antiviral medication for SARS-CoV-2 that acts similarly to oseltamivir phosphate (Tamiflu®), a drug used to shorten the course of the flu in people who’ve had symptoms for less than two days and to prevent the flu in asymptomatic people who may have been exposed to the influenza virus. Yet another major long-term effort of NIH and its partners will be to develop safe and effective antiviral medications that work against all coronaviruses, even those with variant genomes. (And, yes, such drugs might even cure the common cold!)
So, while our ACTIV partners and many other researchers around the globe continue to harness the power of science to end the devastating COVID-19 pandemic as soon as possible, we must also consider the lessons learned this past year, in order to prepare ourselves to respond more swiftly to future outbreaks of coronaviruses and other infectious disease threats. Our work is clearly a marathon, not a sprint.
Links:
Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV) (NIH)
COVID-19 Research (NIH)
Combat COVID (U.S. Department of Health and Human Services, Washington, D.C.)
Pull Up a Chair with Dr. Freire: The COVID Conversations (Foundation for the National Institutes of Health, Bethesda, MD)
SARS-COV-2 Antiviral Therapeutics Summit Report, November 2020 (NIH)
Share this:
- Click to share on LinkedIn (Opens in new window)
- Click to share on Pinterest (Opens in new window)
- Click to share on Tumblr (Opens in new window)
- Click to share on Reddit (Opens in new window)
- Click to share on Telegram (Opens in new window)
- Click to share on WhatsApp (Opens in new window)
- Click to print (Opens in new window)
Posted In: News
Tags: Accelerating COVID-19 Therapeutic Interventions and Vaccines, ACTIV, antiviral, apixaban, aspirin, AstraZeneca, AZD7442, Big Effect Trial, blood clots, blood thinner, Boehringer-Ingelheim, Brii Bioscience, BRII-196, BRII-198, camostat mesilate, clinical trials, coronavirus, COVID-19, COVID-19 Clinical & Preclinical Candidate Compound Portal, COVID-19 treatment, COVID-19 vaccine, dexamethasone, drug development, Eli Lilly and Company, EUA, GSK/Vir Biotechnology, heparin, host-targeted immune modulator, Humanigen, immune modulator, lenzilumab, LY-CoV555, monoclonal antibody, novel coronavirus, oseltamivir phosphate, pandemic, public-private partnership, Regeneron, remdesivir, risankizumab, SARS-CoV-2, Tamiflu, therapeutics, TMPRSS2, treatments, VIR-7831
Charting a Rapid Course Toward Better COVID-19 Tests and Treatments
Posted on by Dr. Francis Collins

It is becoming apparent that our country is entering a new and troubling phase of the pandemic as SARS-CoV-2, the novel coronavirus that causes COVID-19, continues to spread across many states and reaches into both urban and rural communities. This growing community spread is hard to track because up to 40 percent of infected people seem to have no symptoms. They can pass the virus quickly and unsuspectingly to friends and family members who might be more vulnerable to becoming seriously ill. That’s why we should all be wearing masks when we go out of the house—none of us can be sure we’re not that asymptomatic carrier of the virus.
This new phase makes fast, accessible, affordable diagnostic testing a critical first step in helping people and communities. In recognition of this need, NIH’s Rapid Acceleration of Diagnostics (RADx) initiative, just initiated in late April, has issued an urgent call to the nation’s inventors and innovators to develop fast, easy-to-use tests for SARS-CoV-2, the novel coronavirus that causes COVID-19. It brought a tremendous response, and NIH selected about 100 of the best concepts for an intense one-week “shark-tank” technology evaluation process.
Moving ahead at an unprecedented pace, NIH last week announced the first RADx projects to come through the deep dive with flying colors and enter the scale-up process necessary to provide additional rapid testing capacity to the U.S. public. As part of the RADx initiative, seven biomedical technology companies will receive a total of $248.7 million in federal stimulus funding to accelerate their efforts to scale up new lab-based and point-of-care technologies.
Four of these projects will aim to bolster the nation’s lab-based COVID-19 diagnostics capacity by tens of thousands of tests per day as soon as September and by millions by the end of the year. The other three will expand point-of-care testing for COVID-19, making results more rapidly and readily available in doctor’s offices, urgent care clinics, long-term care facilities, schools, child care centers, or even at home.
This is only a start, and we expect that more RADx projects will advance in the coming months and begin scaling up for wide-scale use. In the meantime, here’s an overview of the first seven projects developed through the initiative, which NIH is carrying out in partnership with the Office of the Assistant Secretary of Health, the Biomedical Advanced Research and Development Authority, and the Department of Defense:
Point-of-Care Testing Approaches
Mesa Biotech. Hand-held testing device detects the genetic material of SARS-CoV-2. Results are read from a removable, single-use cartridge in 30 minutes.
Quidel. Test kit detects protein (viral antigen) from SARS-CoV-2. Electronic analyzers provide results within 15 minutes. The U.S. Department of Health and Human Service has identified this technology for possible use in nursing homes.
Talis Biomedical. Compact testing instrument uses a multiplexed cartridge to detect the genetic material of SARS-CoV-2 through isothermal amplification. Optical detection system delivers results in under 30 minutes.
Lab-based Testing Approaches
Ginkgo Bioworks. Automated system uses next-generation sequencing to scan patient samples for SARS-CoV-2’s genetic material. This system will be scaled up to make it possible to process tens of thousands of tests simultaneously and deliver results within one to two days. The company’s goal is to scale up to 50,000 tests per day in September and 100,000 per day by the end of 2020.
Helix OpCo. By combining bulk shipping of test kits and patient samples, automation, and next-generation sequencing of genetic material, the company’s goal is to process up to 50,000 samples per day by the end of September and 100,000 per day by the end of 2020.
Fluidigm. Microfluidics platform with the capacity to process thousands of polymerase chain reaction (PCR) tests for SARS-CoV-2 genetic material per day. The company’s goal is to scale up this platform and deploy advanced integrated fluidic chips to provide tens to hundreds of thousands of new tests per day in the fall of 2020. Most tests will use saliva.
Mammoth Biosciences. System uses innovative CRISPR gene-editing technology to detect key pieces of SARS-CoV-2 genetic material in patient samples. The company’s goal is to provide a multi-fold increase in testing capacity in commercial laboratories.
At the same time, on the treatment front, significant strides continue to be made by a remarkable public-private partnership called Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV). Since its formation in May, the partnership, which involves 20 biopharmaceutical companies, academic experts, and multiple federal agencies, has evaluated hundreds of therapeutic agents with potential application for COVID-19 and prioritized the most promising candidates.
Among the most exciting approaches are monoclonal antibodies (mAbs), which are biologic drugs derived from neutralizing antibodies isolated from people who’ve survived COVID-19. This week, the partnership launched two trials (one for COVID-19 inpatients, the other for COVID-19 outpatients) of a mAB called LY-CoV555, which was developed by Eli Lilly and Company, Indianapolis, IN. It was discovered by Lilly’s development partner AbCellera Biologics Inc. Vancouver, Canada, in collaboration with the NIH’s National Institute of Allergy and Infectious Diseases (NIAID). In addition to the support from ACTIV, both of the newly launched studies also receive support for Operation Warp Speed, the government’s multi-agency effort against COVID-19.
LY-CoV555 was derived from the immune cells of one of the very first survivors of COVID-19 in the United States. It targets the spike protein on the surface of SARS-CoV-2, blocking it from attaching to human cells.
The first trial, which will look at both the safety and efficacy of the mAb for treating COVID-19, will involve about 300 individuals with mild to moderate COVID-19 who are hospitalized at facilities that are part of existing clinical trial networks. These volunteers will receive either an intravenous infusion of LY-CoV555 or a placebo solution. Five days later, their condition will be evaluated. If the initial data indicate that LY-CoV555 is safe and effective, the trial will transition immediately—and seamlessly—to enrolling an additional 700 participants with COVID-19, including some who are severely ill.
The second trial, which will evaluate how LY-CoV555 affects the early course of COVID-19, will involve 220 individuals with mild to moderate COVID-19 who don’t need to be hospitalized. In this study, participants will randomly receive either an intravenous infusion of LY-CoV555 or a placebo solution, and will be carefully monitored over the next 28 days. If the data indicate that LY-CoV555 is safe and shortens the course of COVID-19, the trial will then enroll an additional 1,780 outpatient volunteers and transition to a study that will more broadly evaluate its effectiveness.
Both trials are later expected to expand to include other experimental therapies under the same master study protocol. Master protocols allow coordinated and efficient evaluation of multiple investigational agents at multiple sites as the agents become available. These protocols are designed with a flexible, rapidly responsive framework to identify interventions that work, while reducing administrative burden and cost.
In addition, Lilly this week started a separate large-scale safety and efficacy trial to see if LY-CoV555 can be used to prevent COVID-19 in high-risk residents and staff at long-term care facilities. The study isn’t part of ACTIV.
NIH-funded researchers have been extremely busy over the past seven months, pursuing every avenue we can to detect, treat, and, ultimately, end this devasting pandemic. Far more work remains to be done, but as RADx and ACTIV exemplify, we’re making rapid progress through collaboration and a strong, sustained investment in scientific innovation.
Links:
Coronavirus (COVID-19) (NIH)
Rapid Acceleration of Diagnostics (RADx)
Video: NIH RADx Delivering New COVID-19 Testing Technologies to Meet U.S. Demand (YouTube)
Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV)
Explaining Operation Warp Speed (U.S. Department of Health and Human Resources/Washington, D.C.)
“NIH delivering new COVID-19 testing technologies to meet U.S. demand,” NIH news release,” July 31, 2020.
“NIH launches clinical trial to test antibody treatment in hospitalized COVID-19 patients,” NIH new release, August 4, 2020.
“NIH clinical trial to test antibodies and other experimental therapeutics for mild and moderate COVID-19,” NIH news release, August 4, 2020.
Share this:
- Click to share on LinkedIn (Opens in new window)
- Click to share on Pinterest (Opens in new window)
- Click to share on Tumblr (Opens in new window)
- Click to share on Reddit (Opens in new window)
- Click to share on Telegram (Opens in new window)
- Click to share on WhatsApp (Opens in new window)
- Click to print (Opens in new window)
Posted In: News
Tags: AbCellerra Biologics, Accelerating COVID-19 Therapeutic Interventions and Vaccines, ACTIV, antibodies, coronavirus, COVID-19, COVID-19 testing, COVID-19 treatment, diagnostics, Eli Lilly and Company, Fluidigm, Gingko Bioworks, Helix OpCo, lab-based testing, LY-CoV555, mAbs, Mammoth Biosciences, master protocols, Mesa Biotech, monoclonal antibody, novel coronavirus, Operation Warp Speed, pandemic, point-of-care tests, Quidel, RADx, Rapid Acceleration of Diagnostics Initiative, saliva test, SARS-CoV-2, spike protein, Talis Biomedical, therapeutics
Drug Discovery from A to Z … Arrhythmias to Zebrafish!
Posted on by Dr. Francis Collins
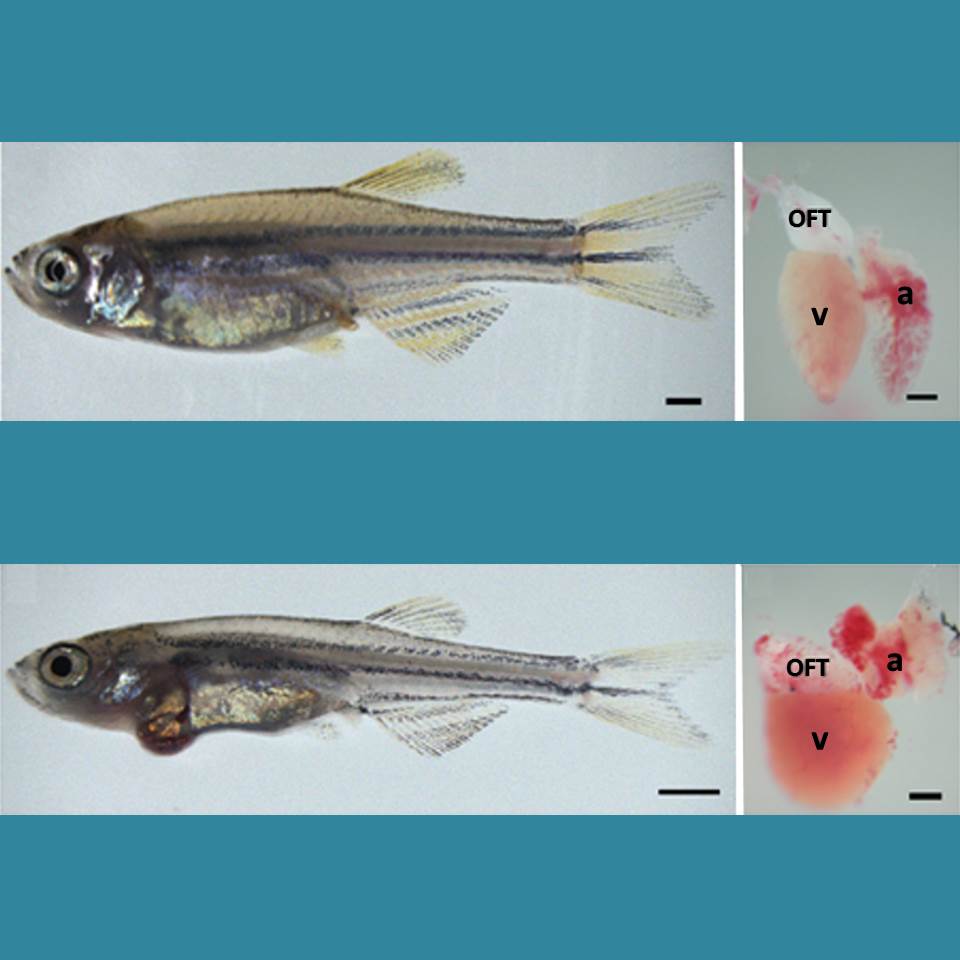
Caption: Healthy zebrafish (top) compared to zebrafish with arrhythmia-causing mutation (bottom). Their hearts are shown to the right, with enlargement indicating a weaker heart. The heart’s outflow tract is marked OFT; atrium, a; and ventricle, v.
Credit: Asimaki et al. Science Translational Medicine
Arrhythmia is a condition in which the heart loses its regular rhythm, beating either too rapidly or too slowly. Occasional irregular heartbeats are harmless, but if sustained they can cause dizziness, fainting, and even sudden death. There are a number of drugs available that can prevent arrhythmias, but none are perfect. Implanted devices can help—pacemakers can keep the heart from beating too slowly, and defibrillators can reset the heart’s rhythm with an electrical shock if a dangerously rapid rhythm develops.
But new treatments are needed. Now, an NIH-funded research team has created an animal model that is advancing efforts to find new drugs to prevent arrhythmia. Led by Jeffrey Saffitz at Beth Israel Deaconess Medical Center, Boston, researchers used genetic engineering techniques to produce zebrafish with genetic mutations identical to those in some people who suffer from a rare inherited disease called arrhythmogenic cardiomyopathy (ACM). In humans, ACM leads to dangerous arrhythmias that can cause sudden cardiac death, usually in people under the age of 35.
Share this:
- Click to share on LinkedIn (Opens in new window)
- Click to share on Pinterest (Opens in new window)
- Click to share on Tumblr (Opens in new window)
- Click to share on Reddit (Opens in new window)
- Click to share on Telegram (Opens in new window)
- Click to share on WhatsApp (Opens in new window)
- Click to print (Opens in new window)
Posted In: Science
Tags: ACM, animal models, arrhythmia, heart, plakoglobin gene, rrhythmogenic cardiomyopathy, SB216763, small molecules, therapeutics, zebrafish
Making This A Land for You and Me
Posted on by Dr. Francis Collins
Today is International Rare Disease Day. In honor of the occasion, I’d like to pay tribute to a few real-life heroes whose struggles have forever changed the landscape of rare disease research.
Folk singer Woody Guthrie is best known for his song, “This Land Is Your Land.” Written more than 70 years ago, “This Land” has taken its place among our nation’s great anthems, setting forth a vision of inclusiveness that has inspired generations of Americans to “sing along.” But the last couple of verses are often omitted. Here’s a version of one of them:
As I was walkin’—I saw a sign there
And that sign said—no trespassin’
But on the other side … it didn’t say nothin’!
Now that side was made for you and me!
These verses brought into the foreground those whom society had marginalized. “This Land” reminded us of their existence, challenged us to live up to our ideals—and include all people in our best vision of ourselves.
Woody performing one version of “This Land”:
Even as he was singing about inclusiveness, Woody Guthrie was starting a long battle against a disease that increasingly cast him outside mainstream society: Huntington’s disease. In most cases—and as was indeed the case for Woody—symptoms of Huntington’s disease do not appear until adulthood. Gradually, this rare, inherited neurological disorder seizes control of its sufferer’s body, mind—and even voice. In 1965, 13 years after he was diagnosed, Woody fell mute. He had long since lost his ability to play guitar. Two years later, he died at the age of 55.
Share this:
- Click to share on LinkedIn (Opens in new window)
- Click to share on Pinterest (Opens in new window)
- Click to share on Tumblr (Opens in new window)
- Click to share on Reddit (Opens in new window)
- Click to share on Telegram (Opens in new window)
- Click to share on WhatsApp (Opens in new window)
- Click to print (Opens in new window)

